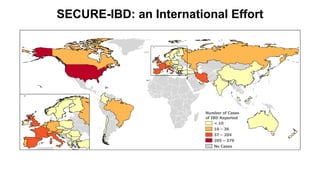
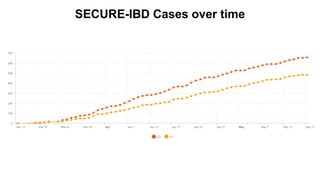
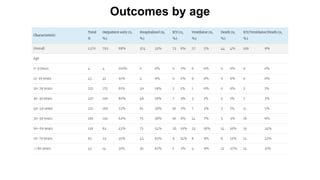
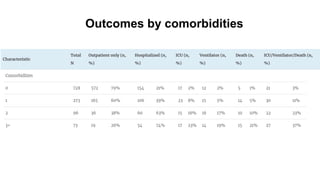
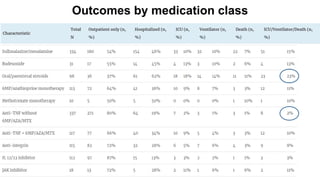
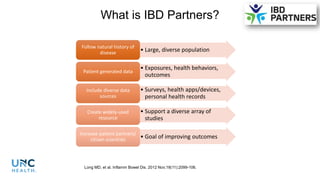
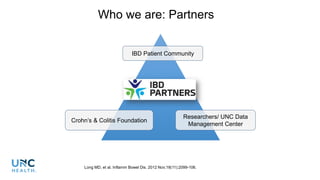
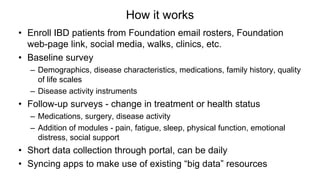
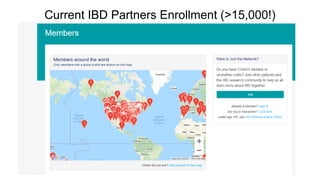
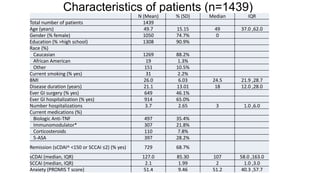
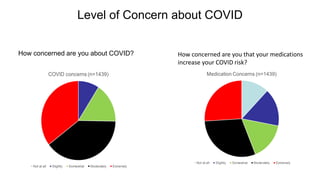
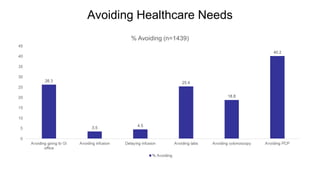
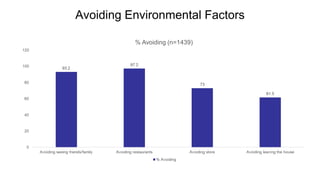
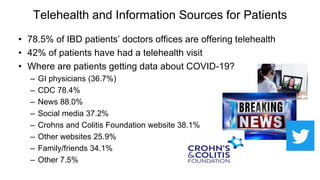
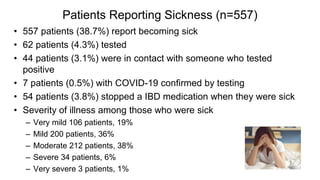
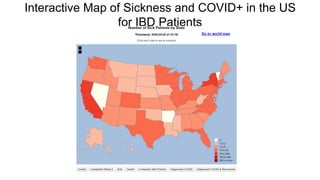
The document provides an update on the Secure-IBD registry, aimed at studying the impact of COVID-19 on patients with inflammatory bowel disease (IBD) through a global database of nearly 1,200 reported cases. It highlights key findings, such as low mortality rates among IBD patients during the pandemic and emphasizes the importance of continuous IBD therapy. Additionally, it outlines the role of patient involvement in research and the ongoing need for data to inform the IBD community about treatment risks and health management during COVID-19.