Batterie
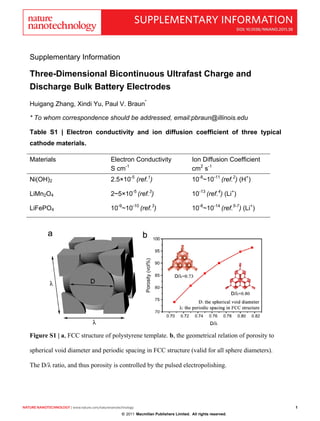
- 1. SUPPLEMENTARY INFORMATION doi: 10.1038/nnano.2011.38 Supplementary Information Three-Dimensional Bicontinuous Ultrafast Charge and Discharge Bulk Battery Electrodes Huigang Zhang, Xindi Yu, Paul V. Braun* * To whom correspondence should be addressed, email:pbraun@illinois.edu Table S1 | Electron conductivity and ion diffusion coefficient of three typical cathode materials. Materials Electron Conductivity Ion Diffusion Coefficient S cm-1 cm2 s-1 Ni(OH)2 2.5×10-5 (ref.1) 10-8~10-11 (ref.2) (H+) LiMn2O4 2~5×10-5 (ref.3) 10-13 (ref.4) (Li+) LiFePO4 10-9~10-10 (ref.3) 10-8~10-14 (ref.5-7) (Li+) Figure S1 | a, FCC structure of polystyrene template. b, the geometrical relation of porosity to spherical void diameter and periodic spacing in FCC structure (valid for all sphere diameters). The D/λ ratio, and thus porosity is controlled by the pulsed electropolishing. nature nanotechnology | www.nature.com/naturenanotechnology 1 1 © 2011 Macmillan Publishers Limited. All rights reserved.
- 2. SUPPLEMENTARY INFORMATION doi: 10.1038/nnano.2011.38 b O Intensity (a.u.) Ni Ni 0.0 0.5 1.0 1.5 2.0 Energy (KeV) c Nickel Oxyhydroxide (i) * * JCPDS No. 59-0464 # Ni Foam Intensity (a.u.) (ii) * # (iii) * # 10 15 20 25 30 35 40 2(deg) Figure S2 | a, TEM image and b, EDX spectrum of the electrodeposited nickel oxyhydroxide. c XRD patterns of (i) the cathode electrode on the Au/Cr-coated glass substrate, (ii) the active material on nickel foam, (iii) nickel foam. The EDX results of the active material on the NiOOH cathode only show nickel and oxygen peaks. The thin film of the electrodeposited nickel oxyhydroxide usually has very weak diffraction peaks just as the previous reports disclosed.8,9 Our NiOOH cathode shows the diffraction peak of nickel oxyhydroxide (JCPDS card #59-0464), However the peak is very broad. In order to exclude the possibility of glass background diffraction, we electrodeposited active material on the pure nickel foam. After comparing the XRD patterns of the nickel foam 2 2 nature nanotechnology | www.nature.com/naturenanotechnology © 2011 Macmillan Publishers Limited. All rights reserved.
- 3. doi: 10.1038/nnano.2011.38 SUPPLEMENTARY INFORMATION with and without active material deposition, it can be concluded that the broad peak results from the active material. The very broad shape of the diffraction peaks of NiOOH/Ni(OH)2 electrode materials has been reported previously due to the size and defect effects,10-12 which is correlated to the high electrochemical activity.10,11,13,14 a b Lithium Manganese Oxide Mn 2p 3/2 * JCPDS No. 35-0782 * # Nickel JCPDS No. 04-0850 Mn 2p 1/2 Intensity (a.u.) * # * # 10 20 30 40 50 60 70 660 650 640 deg Binding Energy (eV) Figure S3 | a, X-ray diffraction pattern of lithiated MnO2 electrodes. b, X-ray photoelectron spectrum of Mn 2p of lithiated MnO2 electrode. The XRD pattern of the lithiated MnO2 electrode can be indexed to the JCPDS card #35-0782 (lithium manganese oxide). The XPS peak positions and the intensity ratio of Mn 2p3/2 and Mn 2p1/2 show the synthesized lithium MnO2 closely resemble the spinel material.15 Although the XRD and XPS data show the appearance of a spinel phase, the discharge curves did not exhibit the two flat stages which the well-crystallized spinel LiMn2O4 usually shows. This may be due to the low-temperature synthesis which is in agreement with previous report of low-temperature molten salt synthesis of lithium manganese oxide.16 3 nature nanotechnology | www.nature.com/naturenanotechnology 3 © 2011 Macmillan Publishers Limited. All rights reserved.
- 4. SUPPLEMENTARY INFORMATION doi: 10.1038/nnano.2011.38 300 280 Capacity (mA h g ) -1 260 240 220 0 20 40 60 80 100 Cycle Number Figure S4 | Capacity of the NiOOH electrode as a function of cycle number. Discharge and charge rates are ~6C. Figure S5 | a, Cross-sectional SEM image of the MnO2 cathode. b, Lithiated MnO2 cathode. 4 4 nature nanotechnology | www.nature.com/naturenanotechnology © 2011 Macmillan Publishers Limited. All rights reserved.
- 5. doi: 10.1038/nnano.2011.38 SUPPLEMENTARY INFORMATION 4.5 4.0 Potential (V vs Li/Li ) + 3.5 3.0 0.9C 3C 2.5 62C 308C 615C 2.0 0.0 0.2 0.4 0.6 0.8 1.0 Capacity Retention Figure S6 | The discharge curves of the sample of lithiated MnO2 cathode with a lithiated MnO2 layer thickness of 150~200 nm. 200 180 Capacity (mA h g ) -1 160 140 120 100 0 10 20 30 40 50 Cycle Number Figure S7 | Capacity of the MnO2 electrode as a function of cycle number. Discharge and charge rates are ~3C. 5 nature nanotechnology | www.nature.com/naturenanotechnology 5 © 2011 Macmillan Publishers Limited. All rights reserved.
- 6. SUPPLEMENTARY INFORMATION doi: 10.1038/nnano.2011.38 Other Potential Battery Chemistries Porous nickel as the current collector in nickel hydrogen batteries has been used in aerospace since 1970s17. Via similar electrodeposition routes as used to form NiOOH-based electrodes in both this early work and our work, CoOOH and MnOOH-based electrodes18-20 could deposited onto our porous scaffold structure to form a bicontinuous electrode. In a Li-ion battery the current collector needs to withstand a large potential range, generally outside the thermodynamic stability range of the current collector. Even a pure Ni current collector can withstand the typical operating conditions through formation of surface layer, and if alloyed with chromium, can resist even a 5 V potential21; Ni-Cr alloys can easily be electroplated. In the literature, Ni has been successfully reported as the current collector for a range of cathode materials22 including V2O523, V6O1324, metal sulphides, lithium manganese oxide25, and MoO326. If desired, these materials could be conformally deposited onto our porous scaffold structure to form a bicontinuous electrode. Supplementary Preparation Details The preparation of macroporous Ni can be referred to our previous paper27. A silicon wafer (Montco Silicon Technologies, Inc.) or glass was cleaned with piranha and coated with 5nm chromium and 50~100 nm gold by e-beam evaporation (Temescal, Inc). The substrate obtained was submerged in an aqueous solution of 3-mercapto-1-propanesulfonic acid, sodium salt (SigmaAldrich Corp.) for 2 h. Polystyrene spheres with the diameter of 1.8 μm or 466 nm (Molecular Probes) were dispersed in deionized water to prepare a 0.5~2 wt% suspension. After drying with blown air, the substrate was placed vertically into a vial with the PS suspension at 50~55 °C for deposition (SI Fig. 8a). 6 6 nature nanotechnology | www.nature.com/naturenanotechnology © 2011 Macmillan Publishers Limited. All rights reserved.
- 7. doi: 10.1038/nnano.2011.38 SUPPLEMENTARY INFORMATION After annealing at 95 °C for 2 h, the opal sample was electrodeposited with the current of ~2 mA cm-2 in a commercial electroplating solution (Techni Nickel S, Technic Corp.) as shown in SI Fig. 8b. The sample obtained was then cleaned, dried and soaked into tetrahydrofuran (SigmaAldrich Corp.) to remove PS. a b c d + 80ms - - 1.5V -2 2mA cm 0.7V - + + Figure S8 | a, preparation of opal templates by vertical deposition. b, nickel plating into the opal template. c, pulsed electropolish nickel inverse opal. d, pulsed electrodeposition of nickel oxyhydroxide and manganese oxide. The etching solution containing 0.5~1 M Ni2+ was prepared by electrochemically dissolving Ni metal into EP1250 (Technic Corp) at 6 V. The macroporous nickel was electropolished in this solution by 6 V pulses (80 ms on and 16 s off) at 60°C as shown in SI Fig. 8c. The cycle number is controlled by a potentiostat (Model 263A, Princeton Applied Research) with Power Pulse Software. Active materials are electrodeposited by pulsed potential technique as shown in SI Fig. 8d. Rest time after each pulse is provided for mass transport to avoid non- uniform deposition. 7 nature nanotechnology | www.nature.com/naturenanotechnology 7 © 2011 Macmillan Publishers Limited. All rights reserved.
- 8. SUPPLEMENTARY INFORMATION doi: 10.1038/nnano.2011.38 References 1. Srinivasan, V., Weidner, J. W. & White, R. E. Mathematical models of the nickel hydroxide active material. J. Solid State Electrochem. 4, 367-382 (2000). 2. Paxton, B. & Newman, J. Modeling of nickel/metal hydride batteries. J. Electrochem. Soc. 144, 3818-3831 (1997). 3. Chung, S. Y., Bloking, J. T. & Chiang, Y. M. Electronically conductive phospho-olivines as lithium storage electrodes. Nature Mater. 1, 123-128 (2002). 4. Deiss, E. Spurious chemical diffusion coefficients of Li+ in electrode materials evaluated with GITT. Electrochim. Acta 50, 2927-2932 (2005). 5. Morgan, D., Van der Ven, A. & Ceder, G. Li conductivity in LixMPO4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochem. Solid State Lett. 7, A30-A32 (2004). 6. Xie, J. et al. Li-ion diffusion kinetics in LiFePO4 thin film prepared by radio frequency magnetron sputtering. Electrochim. Acta 54, 4631-4637 (2009). 7. Churikov, A. V. et al. Determination of lithium diffusion coefficient in LiFePO4 electrode by galvanostatic and potentiostatic intermittent titration techniques. Electrochim. Acta 55, 2939-2950 (2010). 8. Wu, M. S., Yang, C. H. & Wang, M. J. Morphological and structural studies of nanoporous nickel oxide films fabricated by anodic electrochemical deposition techniques. Electrochim. Acta 54, 155-161 (2008). 9. Wu, M. S., Huang, Y. A. & Yang, C. H. Capacitive behavior of porous nickel oxide/hydroxide electrodes with interconnected nanoflakes synthesized by anodic electrodeposition. J. Electrochem. Soc. 155, A798-A805 (2008). 10. Kiani, M. A., Mousavi, M. F. & Ghasemi, S. Size effect investigation on battery performance: Comparison between micro- and nano-particles of beta-Ni(OH)2 as nickel battery cathode material. J. Power Sources 195, 5794-5800 (2010). 11. Delmas, C. & Tessier, C. Stacking faults in the structure of nickel hydroxide: a rationale of its high electrochemical activity. J. Mater. Chem. 7, 1439-1443 (1997). 12. Ramesh, T. N. X-ray Diffraction Studies on the Thermal Decomposition Mechanism of Nickel Hydroxide. J. Phys. Chem. B 113, 13014-13017 (2009). 13. Watanabe, K., Kikuoka, T. & Kumagai, N. Physical and electrochemical characteristics of nickel hydroxide as a positive material for rechargeable alkaline batteries. J. Appl. Electrochem. 25, 219-226 (1995). 14. Bernard, M. C. et al. Structural defects and electrochemical reactivity of beta-Ni(OH)(2). J. Power Sources 63, 247-254 (1996). 15. Ramana, C. V., Massot, M. & Julien, C. M. XPS and Raman spectroscopic characterization of LiMn2O4 spinels. Surface and Interface Analysis 37, 412-416 (2005). 16. Reimers, J. N., Fuller, E. W., Rossen, E. & Dahn, J. R. Synthesis and electrochemical studies of LiMnO2 prepared of low-temperatures. J. Electrochem. Soc. 140, 3396-3401 (1993). 17. Zimmerman, A. H. Nickel-hydrogen batteries principles and practice. (American institute of aeronautics and astronautics, Inc., 2009). 18. Cao, L., Xu, F., Liang, Y. Y. & Li, H. L. Preparation of the Novel Nanocomposite Co(OH)2/ Ultra-Stable Y Zeolite and Its Application as a Supercapacitor with High Energy Density. Adv. Mater. 16, 1853-1857 (2004). 8 8 nature nanotechnology | www.nature.com/naturenanotechnology © 2011 Macmillan Publishers Limited. All rights reserved.
- 9. doi: 10.1038/nnano.2011.38 SUPPLEMENTARY INFORMATION 19. Yang, G. W., Xu, C. L. & Li, H. L. Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem. Commun., 6537-6539 (2008). 20. El-Deab, M. S. & Ohsaka, T. Electrosynthesis of single-crystalline MnOOH nanorods onto Pt electrodes - Electrocatalytic activity toward reduction of oxygen. J. Electrochem. Soc. 155, D14-D21 (2008). 21. Yao, M. et al. High-Capacity Electric Double Layer Capacitor Using Three-Dimensional Porous Current Collector. Electrochem. Solid State Lett. 10, A245-A249 (2007). 22. Chang, O.-K., Hall, J. C., Phillips, J. & Silvester, L. F. Positive current collector for lithium secondary system. US patent US4892796 (1990). 23. Parent, M. J., Passerini, S., Owens, B. B. & Smyrl, W. H. Composites of V2O5 Aerogel and Nickel Fiber as High Rate Intercalation Electrodes. J. Electrochem. Soc. 146, 1346- 1350 (1999). 24. West, K., Zachau-Christiansen, B. & Jacobsen, T. Electrochemical properties of non- stoichiometric V6O13. Electrochim. Acta 28, 1829-1833 (1983). 25. Macklin, W. J., Neat, R. J. & Powell, R. J. Performance of lithium manganese oxide spinel electrodes in a lithium polymer electrolyte cell. J. Power Sources 34, 39-49 (1991). 26. Whitehead, A. H. & Schreiber, M. Current Collectors for Positive Electrodes of Lithium- Based Batteries. J. Electrochem. Soc. 152, A2105-A2113 (2005). 27. Yu, X. D., Lee, Y. J., Furstenberg, R., White, J. O. & Braun, P. V. Filling fraction dependent properties of inverse opal metallic photonic crystals. Adv. Mater. 19, 1689- 1692 (2007). 9 nature nanotechnology | www.nature.com/naturenanotechnology 9 © 2011 Macmillan Publishers Limited. All rights reserved.
