Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Measurement of conductance and kohlrausch's law.pdf

Measurement of conductance and kohlrausch's law.pdf
Viewers also liked
Viewers also liked (8)
More from twindsor1
More from twindsor1 (20)
States of Matter and physical and chemical changes

States of Matter and physical and chemical changes
Conductivity of solutions
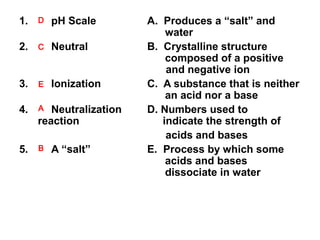
- 1. 1. D pH Scale 2. C Neutral 3. E Ionization 4. A Neutralization reaction 5. B A “salt” A. Produces a “salt” and water B. Crystalline structure composed of a positive and negative ion C. A substance that is neither an acid nor a base D. Numbers used to indicate the strength of acids and bases E. Process by which some acids and bases dissociate in water
- 2. Review Acids Bases pH Testing Acids/Bases Neutralization reactions
- 3. Conductivity of Solutions When an acid, a base, or a salt dissolves in water Their atoms don’t stay together, they unlock When they unlock they don’t stay neutral
- 4. Conductivity of Solutions They become charged (+) or (-) Ions Ions let electricity pass through
- 6. Come over here and give me some sugar…
- 7. Conductivity of Solutions Atoms of alcohol, sugar, distilled water, don’t unlock Don’t form ions Don’t conduct electricity