Log - OR Temperature and Humidity Log
•Download as DOCX, PDF•
1 like•4,610 views
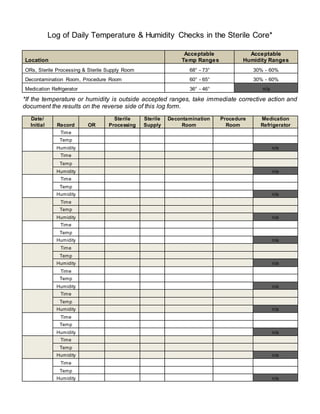
This document logs the daily temperature and humidity checks of several sterile core locations, including operating rooms, sterile processing, sterile supply room, decontamination room, procedure room, and medication refrigerator. It records the acceptable temperature and humidity ranges for each location and provides space to record the time, temperature, and humidity readings from daily checks to ensure standards are maintained. Any readings outside the accepted ranges require corrective action to be taken and documented on the reverse side.
Report
Share
Report
Share
Recommended
FDA Compliance Programme Manual : Program 7356.002A is a manual which FDA investigators follow while inspection Sterile drug manufacturing operations This guidance for FDA Investigators covers the manufacture and testing of all sterile drug products, including Drugs that are sterilized by filtration or other means, aseptically processed, and drug products that are terminally sterilized.
The type of products covered by this program include
Sterile bulk drugs
Ophthalmic drugs
Otic dosage forms
Small volume parenteral (SVS)
Products for small molecule and licensed biological therapeutic drug products,
Large volume parenteral (LVP) products, and
Any other drug products required to be sterile or labeled as sterile.
Excluded under this Programme
Center for Biologics Evaluation and Research (CBER) regulated products
Veterinary drug product
At the end of this compliance guide 7356.002A questions have been given to help FDA investigators understand the manufacturing operations better and help in conduct of Inspections. Subsequent slides gives summary of these questions, which we feel, will help us prepare for an Inspection.Questions FDA will ask during Inspection of Sterile Drug Manufacturing.

Questions FDA will ask during Inspection of Sterile Drug Manufacturing.GMP EDUCATION : Not for Profit Organization
Recommended
FDA Compliance Programme Manual : Program 7356.002A is a manual which FDA investigators follow while inspection Sterile drug manufacturing operations This guidance for FDA Investigators covers the manufacture and testing of all sterile drug products, including Drugs that are sterilized by filtration or other means, aseptically processed, and drug products that are terminally sterilized.
The type of products covered by this program include
Sterile bulk drugs
Ophthalmic drugs
Otic dosage forms
Small volume parenteral (SVS)
Products for small molecule and licensed biological therapeutic drug products,
Large volume parenteral (LVP) products, and
Any other drug products required to be sterile or labeled as sterile.
Excluded under this Programme
Center for Biologics Evaluation and Research (CBER) regulated products
Veterinary drug product
At the end of this compliance guide 7356.002A questions have been given to help FDA investigators understand the manufacturing operations better and help in conduct of Inspections. Subsequent slides gives summary of these questions, which we feel, will help us prepare for an Inspection.Questions FDA will ask during Inspection of Sterile Drug Manufacturing.

Questions FDA will ask during Inspection of Sterile Drug Manufacturing.GMP EDUCATION : Not for Profit Organization
This presentation gives a summary of the WHO guidance on HVAC Systems.WHO Guidance on HVAC Systems for Non Sterile Pharmaceuticals

WHO Guidance on HVAC Systems for Non Sterile PharmaceuticalsGMP EDUCATION : Not for Profit Organization
More Related Content
What's hot
This presentation gives a summary of the WHO guidance on HVAC Systems.WHO Guidance on HVAC Systems for Non Sterile Pharmaceuticals

WHO Guidance on HVAC Systems for Non Sterile PharmaceuticalsGMP EDUCATION : Not for Profit Organization
What's hot (20)
Dispensing Operations in Manufacturing of Pharmaceutcal Products

Dispensing Operations in Manufacturing of Pharmaceutcal Products
Guidance on gloves maintenance in Isolator and RABS

Guidance on gloves maintenance in Isolator and RABS
WHO Guidance on HVAC Systems for Non Sterile Pharmaceuticals

WHO Guidance on HVAC Systems for Non Sterile Pharmaceuticals
Similar to Log - OR Temperature and Humidity Log
Similar to Log - OR Temperature and Humidity Log (8)
Controlling Relative Humidity and Condensation in a CO2 Incubator

Controlling Relative Humidity and Condensation in a CO2 Incubator
Log - OR Temperature and Humidity Log
- 1. Log of Daily Temperature & Humidity Checks in the Sterile Core* Location Acceptable Temp Ranges Acceptable Humidity Ranges ORs, Sterile Processing & Sterile Supply Room 68° - 73° 30% - 60% Decontamination Room, Procedure Room 60° - 65° 30% - 60% Medication Refrigerator 36° - 46° n/a *If the temperature or humidity is outside accepted ranges, take immediate corrective action and document the results on the reverse side of this log form. Date/ Initial Record OR Sterile Processing Sterile Supply Decontamination Room Procedure Room Medication Refrigerator Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a Time Temp Humidity n/a
