Electrons, Electron Orbitals, Molecules, PowerPoint Quiz Game, Challenge
This PowerPoint is one small part of the Atoms and Periodic Table of the Elements unit from www.sciencepowerpoint.com. This unit consists of a five part 2000+ slide PowerPoint roadmap, 12 page bundled homework package, modified homework, detailed answer keys, 15 pages of unit notes for students who may require assistance, follow along worksheets, and many review games. The homework and lesson notes chronologically follow the PowerPoint slideshow. The answer keys and unit notes are great for support professionals. The activities and discussion questions in the slideshow are meaningful. The PowerPoint includes built-in instructions, visuals, and review questions. Also included are critical class notes (color coded red), project ideas, video links, and review games. This unit also includes four PowerPoint review games (110+ slides each with Answers), 38+ video links, lab handouts, activity sheets, rubrics, materials list, templates, guides, and much more. Also included is a 190 slide first day of school PowerPoint presentation. Areas of Focus: -Atoms (Atomic Force Microscopes), Rutherford's Gold Foil Experiment, Cathode Tube, Atoms, Fundamental Particles, The Nucleus, Isotopes, AMU, Size of Atoms and Particles, Quarks, Recipe of the Universe, Atomic Theory, Atomic Symbols, #'s, Valence Electrons, Octet Rule, SPONCH Atoms, Molecules, Hydrocarbons (Structure), Alcohols (Structure), Proteins (Structure), Periodic Table of the Elements, Organization of Periodic Table, Transition Metals, Electron Negativity, Non-Metals, Metals, Metalloids, Atomic Bonds, Ionic Bonds, Covalent Bonds, Metallic Bonds, Ionization, and much more. This unit aligns with the Next Generation Science Standards and with Common Core Standards for ELA and Literacy for Science and Technical Subjects. See preview for more information If you have any questions please feel free to contact me. Thanks again and best wishes. Sincerely, Ryan Murphy M.Ed www.sciencepowerpoint@gmail.com Teaching Duration = 4+ Weeks
Recommended
Recommended
More Related Content
Similar to Electrons, Electron Orbitals, Molecules, PowerPoint Quiz Game, Challenge
Similar to Electrons, Electron Orbitals, Molecules, PowerPoint Quiz Game, Challenge (20)
More from www.sciencepowerpoint.com
More from www.sciencepowerpoint.com (20)
Recently uploaded
Recently uploaded (20)
Electrons, Electron Orbitals, Molecules, PowerPoint Quiz Game, Challenge
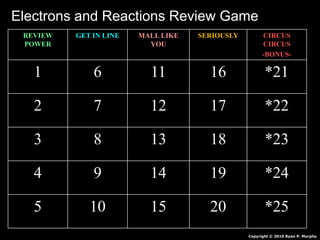
- 1. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 2. • How to play… – Don’t play like Jeo_ _ _ _ y. – Class should be divided into several small groups. – Groups should use science journal (red slide notes), homework, and other available materials to assist you. – Groups can communicate quietly with each other but no sharing answers between groups. • Practice quietly communicating right now? • Practice Communication Question: • Your group gets to order one pizza and you can have two toppings. What does your group want?
- 3. Questions 1-20 = 5pts Each Final Category (Bonus) = 1pt Each Final Questions = 5 pt wager If you wager 5 on the last question and get it wrong you lose 5 pts. Wager 5 and get it right you get 5 pts. Find the Owl = Secretly write “Owl” in the correct box worth 1pt. “I’ll be about this big.”
- 4. • Is your name on the review sheet?
- 5. • Is your name on the review sheet?
- 7. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 8. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 9. • Which is not one of John Daltons Atomic Theories? Copyright © 2010 Ryan P. Murphy
- 10. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. Copyright © 2010 Ryan P. Murphy
- 11. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. Copyright © 2010 Ryan P. Murphy
- 12. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. Copyright © 2010 Ryan P. Murphy
- 13. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have the same type of atoms. Copyright © 2010 Ryan P. Murphy
- 14. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have the same type of atoms. E.) Chemical reactions occur when atoms are rearranged. Copyright © 2010 Ryan P. Murphy
- 15. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have the same type of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 16. • Each Element is made up of one kind of atom. Each type of atoms has an atomic number. – The atomic number is the number of Protons and Electrons in the atom.
- 17. • Atomic Mass Units = The number of protons, neutrons, and electrons in an atom. Copyright © 2010 Ryan P. Murphy
- 19. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy Note: Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
- 20. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 21. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 22. • Please name the elements below? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 23. • Elements want to follow the octet rule which means they want 8 electrons in their outer shell (valence). • A.) 2 • B.) 8 • C.) 64 • D.) 108 • E.) -14 Copyright © 2010 Ryan P. Murphy
- 24. • Elements want to follow the octet rule which means they want 8 electrons in their outer shell (valence). • A.) 2 • B.) 8 • C.) 64 • D.) 108 • E.) -14 Copyright © 2010 Ryan P. Murphy
- 25. • This is the name for when two or more atoms join together chemically.
- 26. • What is the empirical formula of Propane? Copyright © 2010 Ryan P. Murphy C C3H8
- 28. • Please make a Lewis Dot Structure for Methane CH4? Copyright © 2010 Ryan P. Murphy C
- 29. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 30. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 31. • Carbohydrates are made of these three chemical elements? Copyright © 2010 Ryan P. Murphy
- 32. • These are organic compounds consisting entirely of hydrogen and carbon. Copyright © 2010 Ryan P. Murphy
- 33. • These molecules are mostly carbon and hydrogen with an OH group? – Which one is Ethanol C2H6O
- 34. • Name this type of nitrogenous compound? Copyright © 2010 Ryan P. Murphy
- 35. • Please name this molecule? Copyright © 2010 Ryan P. Murphy
- 36. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 37. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 38. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 39. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 40. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 41. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 42. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 43. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 44. • O2 Oxygen Gas can be seen here in a… Copyright © 2010 Ryan P. Murphy
- 45. • O2 Oxygen Gas can be seen here in a… • A.) Single Bond B.) Double Bond • C.) Triple Bond D.) Quaddro Bond • E.) Oxygen cannot bond because it’s negative. • C.) Tr Copyright © 2010 Ryan P. Murphy
- 46. F What is the Electron Configuration of F?
- 48. Can you try? P
- 49. • Name the famous scientists below?
- 50. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 51. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 52. • Name this movie?
- 57. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 58. • Please name the element below? Note: Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
- 60. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 61. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 62. • Which is not one of John Daltons Atomic Theories? Copyright © 2010 Ryan P. Murphy
- 63. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have the same type of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 64. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have the same type of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 65. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have different types of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 66. • Each Element is made up of one kind of atom. Each type of atoms has an atomic number. – The atomic number is the number of Protons and Electrons in the atom.
- 67. • Each Element is made up of one kind of atom. Each type of atoms has an atomic number. – The atomic number is the number of Protons and Electrons in the atom.
- 68. • Each Element is made up of one kind of atom. Each type of atoms has an atomic number. – The atomic number is the number of Protons and Electrons in the atom.
- 69. • Each Element is made up of one kind of atom. Each type of atoms has an atomic number. – The atomic number is the number of Protons and Electrons in the atom.
- 70. • Each Element is made up of one kind of atom. Each type of atoms has an atomic number. – The atomic number is the number of Protons and Electrons in the atom.
- 71. • Atomic Mass Units = The number of protons, neutrons, and electrons in an atom. Copyright © 2010 Ryan P. Murphy
- 72. • Atomic Mass Units = The number of protons, neutrons, and electrons in an atom. Copyright © 2010 Ryan P. Murphy
- 73. • Atomic Mass Units = The number of protons, neutrons, and electrons in an atom. Copyright © 2010 Ryan P. Murphy
- 79. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 80. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 81. • How many electrons are in the first three energy levels? 2, Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 82. • How many electrons are in the first three energy levels? 2, Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 83. • How many electrons are in the first three energy levels? 2, 8, Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 84. • How many electrons are in the first three energy levels? 2, 8, Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 85. • How many electrons are in the first three energy levels? 2, 8, 7 Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 86. • How many electrons are in the first three energy levels? 2, 8, 7 Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
- 87. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 88. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 89. • Please name the elements below? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 90. • Please name the elements below? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 91. • Please name the elements below? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 92. • Elements want to follow the octet rule which means they want 8 electrons in their outer shell (valence). • A.) 2 • B.) 8 • C.) 64 • D.) 108 • E.) -14 Copyright © 2010 Ryan P. Murphy
- 93. • Elements want to follow the octet rule which means they want 8 electrons in their outer shell (valence). • A.) 2 • B.) 8 • C.) 64 • D.) 108 • E.) -14 Copyright © 2010 Ryan P. Murphy
- 94. • Elements want to follow the octet rule which means they want 8 electrons in their outer shell (valence). • A.) 2 • B.) 8 • C.) 64 • D.) 108 • E.) -14 Copyright © 2010 Ryan P. Murphy
- 95. • Elements want to follow the octet rule which means they want 8 electrons in their outer shell (valence). • A.) 2 • B.) 8 • C.) 64 • D.) 108 • E.) -14 Copyright © 2010 Ryan P. Murphy
- 96. • This is the name for when two or more atoms join together chemically.
- 97. • This is the name for when two or more atoms join together chemically.
- 98. • This is the name for when two or more atoms join together chemically.
- 99. • What is the empirical formula of Propane? Copyright © 2010 Ryan P. Murphy C C3H8
- 100. • What is the empirical formula of Propane? Copyright © 2010 Ryan P. Murphy C C3H8
- 101. • What is the empirical formula of Propane? Copyright © 2010 Ryan P. Murphy C C3H8
- 103. • Please make a Lewis Dot Structure for Methane CH4? Copyright © 2010 Ryan P. Murphy C
- 104. • Please make a Lewis Dot Structure for Methane CH4? Copyright © 2010 Ryan P. Murphy C
- 105. • Please make a Lewis Dot Structure for Methane CH4? Copyright © 2010 Ryan P. Murphy C
- 106. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 107. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 108. • Carbohydrates are made of these three chemical elements? Copyright © 2010 Ryan P. Murphy
- 109. • Carbohydrates are made of these three chemical elements? Copyright © 2010 Ryan P. Murphy
- 110. • These are organic compounds consisting entirely of hydrogen and carbon. Copyright © 2010 Ryan P. Murphy
- 111. • These are organic compounds consisting entirely of hydrogen and carbon. Copyright © 2010 Ryan P. Murphy
- 112. • These are organic compounds consisting entirely of hydrogen and carbon. Copyright © 2010 Ryan P. Murphy
- 113. • These molecules are mostly carbon and hydrogen with an OH group? – Which one is Ethanol C2H6O
- 114. • These molecules are mostly carbon and hydrogen with an OH group? – Which one is Ethanol C2H6O
- 115. • These molecules are mostly carbon and hydrogen with an OH group? – Which one is Ethanol C2H6O
- 116. • These molecules are mostly carbon and hydrogen with an OH group? – Which one is Ethanol C2H6O
- 117. • These molecules are mostly carbon and hydrogen with an OH group? – Which one is Ethanol C2H6O
- 118. • These molecules are mostly carbon and hydrogen with an OH group? – Which one is Ethanol C2H6O
- 119. • Name this type of nitrogenous compound? Copyright © 2010 Ryan P. Murphy
- 120. • Name this type of nitrogenous compound? Copyright © 2010 Ryan P. Murphy
- 121. • Name this type of nitrogenous compound? Copyright © 2010 Ryan P. Murphy
- 122. • Name this type of nitrogenous compound? Copyright © 2010 Ryan P. Murphy
- 123. • Please name this molecule? Copyright © 2010 Ryan P. Murphy
- 124. • Please name this molecule? Glucose Copyright © 2010 Ryan P. Murphy
- 125. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 126. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 127. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 128. • This describes that no two electrons in an atom can have identical quantum numbers. Different spins – Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons – A.) Daltons Atomic Laws – B.) Crookes Ideas – C.) Pauli Exclusion Principle – D.) Edwards Motives – E.) Jefferson’s Principle of Spontaneity Copyright © 2010 Ryan P. Murphy
- 129. • O2 Oxygen Gas can be seen here in a… • A.) Single Bond B.) Double Bond • C.) Triple Bond D.) Quaddro Bond • E.) Oxygen cannot bond because it’s negative • C.) Tr Copyright © 2010 Ryan P. Murphy O=O
- 130. • O2 Oxygen Gas can be seen here in a… • A.) Single Bond B.) Double Bond • C.) Triple Bond D.) Quaddro Bond • E.) Oxygen cannot bond because it’s negative • C.) Tr Copyright © 2010 Ryan P. Murphy O=O
- 131. F What is the Electron Configuration of F?
- 132. F What is the Electron Configuration of F? 1S2, 2S2, 2P5,
- 133. F What is the Electron Configuration of F? 1S2, 2S2, 2P5,
- 134. F What is the Electron Configuration of F? 1S2, 2S2, 2P5,
- 135. F What is the Electron Configuration of F? 1S2, 2S2, 2P5,
- 136. F What is the Electron Configuration of F? 1S2, 2S2, 2P5, [He] 2S2 2P5
- 138. Can you try? P Ne
- 139. Can you try? P Ne 1s2 2s2 2p6 3s2 3p3
- 140. Can you try? P Ne 1s2 2s2 2p6 3s2 3p3 [Ne] 3s2 3p3
- 141. • Name the famous scientists below?
- 142. • Name the famous scientists below?
- 143. • Name the famous scientists below?
- 144. • Name the famous scientists below?
- 145. • Name the famous scientists below?
- 146. • Name the famous scientists below?
- 147. • Name the famous scientists below?
- 148. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 149. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 150. • Name this movie?
- 151. • Name this movie?
- 163. REVIEW POWER GET IN LINE MALL LIKE YOU SERIOUSLY CIRCUS CIRCUS -BONUS- 1 6 11 16 *21 2 7 12 17 *22 3 8 13 18 *23 4 9 14 19 *24 5 10 15 20 *25 Copyright © 2010 Ryan P. Murphy Electrons and Reactions Review Game
- 164. • Please name the element below? Note: Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
- 165. • Please name the element below?
- 166. • Please name the element below? 2, 8, 18, 32, 18, 1
- 167. • Please name the element below? 2, 8, 18, 32, 18, 1
- 168. • Please name the element below? 2, 8, 18, 32, 18, 1
- 169. • Please name the element below? 2, 8, 18, 32, 18, 1
- 170. • Please name the element below? 2, 8, 18, 32, 18, 1
- 171. • Please name the element below? 2, 8, 18, 32, 18, 1
- 172. • Please name the element below? 2, 8, 18, 32, 18, 1
- 173. • Please name the element below? 2, 8, 18, 32, 18, 1 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s1
- 174. • Please name the element below? 2, 8, 18, 32, 18, 1 [Xe] 4f14 5d10 6s1 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s1
- 175. • Please name the element below? 2, 8, 18, 32, 18, 1 [Xe] 4f14 5d10 6s1 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s1
- 176. • Please name the element below? 2, 8, 18, 32, 18, 1 [Xe] 4f14 5d10 6s1 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s1
- 177. Questions 1-20 = 5pts Each Final Category (Bonus) = 1pt Each Final Questions = 5 pt wager Find the Owl = Secretly write “Owl” in the correct box worth 1pt.
- 180. http://sciencepowerpoint.com/Atoms_Periodic_Table_of_Elements_Unit.html Areas of Focus within The Atoms and Periodic Table Unit: Atoms (Atomic Force Microscopes), Rutherford’s Gold Foil Experiment, Cathode Tube, Atoms, Fundamental Particles, The Nucleus, Isotopes, AMU, Size of Atoms and Particles, Quarks, Recipe of the Universe, Atomic Theory, Atomic Symbols, #’;s, Valence Electrons, Octet Rule, SPONCH Atoms, Molecules, Hydrocarbons (Structure), Alcohols (Structure), Proteins (Structure), Atomic Bonds, Ionic Bonds, Covalent Bonds, Metallic Bonds, , Precipitation Reactions, Acids and Bases, Electron Negativity, Polar Bonds, Chemical Change, Exothermic Reactions, Endothermic Reactions, Laws Conservation of Matter, Balancing Chemical Equations, Oxidation and Reduction, Periodic Table of the Elements, Organization of Periodic Table, Transition Metals, Acids and Bases, Non-Metals, Metals, Metalloids, Ionization.
- 186. • This PowerPoint roadmap is one small part of my Atoms and Periodic Table Unit. • This unit includes a four part 2000+ slide PowerPoint roadmap. • 13 page bundled homework that chronologically follows slideshow • 14 pages of unit notes with visuals. • 3 PowerPoint review games. • Activity sheets, rubrics, advice page, curriculum guide, materials list, and much more. • http://sciencepowerpoint.com
- 188. • Please visit the links below to learn more about each of the units in this curriculum – These units take me about four years to complete with my students in grades 5-10. Earth Science Units Extended Tour Link and Curriculum Guide Geology Topics Unit http://sciencepowerpoint.com/Geology_Unit.html Astronomy Topics Unit http://sciencepowerpoint.com/Astronomy_Unit.html Weather and Climate Unit http://sciencepowerpoint.com/Weather_Climate_Unit.html Soil Science, Weathering, More http://sciencepowerpoint.com/Soil_and_Glaciers_Unit.html Water Unit http://sciencepowerpoint.com/Water_Molecule_Unit.html Rivers Unit http://sciencepowerpoint.com/River_and_Water_Quality_Unit.html = Easier = More Difficult = Most Difficult 5th – 7th grade 6th – 8th grade 8th – 10th grade
- 189. Physical Science Units Extended Tour Link and Curriculum Guide Science Skills Unit http://sciencepowerpoint.com/Science_Introduction_Lab_Safety_Metric_Methods.html Motion and Machines Unit http://sciencepowerpoint.com/Newtons_Laws_Motion_Machines_Unit.html Matter, Energy, Envs. Unit http://sciencepowerpoint.com/Energy_Topics_Unit.html Atoms and Periodic Table Unit http://sciencepowerpoint.com/Atoms_Periodic_Table_of_Elements_Unit.html Life Science Units Extended Tour Link and Curriculum Guide Human Body / Health Topics http://sciencepowerpoint.com/Human_Body_Systems_and_Health_Topics_Unit.html DNA and Genetics Unit http://sciencepowerpoint.com/DNA_Genetics_Unit.html Cell Biology Unit http://sciencepowerpoint.com/Cellular_Biology_Unit.html Infectious Diseases Unit http://sciencepowerpoint.com/Infectious_Diseases_Unit.html Taxonomy and Classification Unit http://sciencepowerpoint.com/Taxonomy_Classification_Unit.html Evolution / Natural Selection Unit http://sciencepowerpoint.com/Evolution_Natural_Selection_Unit.html Botany Topics Unit http://sciencepowerpoint.com/Plant_Botany_Unit.html Ecology Feeding Levels Unit http://sciencepowerpoint.com/Ecology_Feeding_Levels_Unit.htm Ecology Interactions Unit http://sciencepowerpoint.com/Ecology_Interactions_Unit.html Ecology Abiotic Factors Unit http://sciencepowerpoint.com/Ecology_Abiotic_Factors_Unit.html
- 191. • The entire four year curriculum can be found at... http://sciencepowerpoint.com/ Please feel free to contact me with any questions you may have. Thank you for your interest in this curriculum. Sincerely, Ryan Murphy M.Ed www.sciencepowerpoint@gmail.com
