2 data 2011
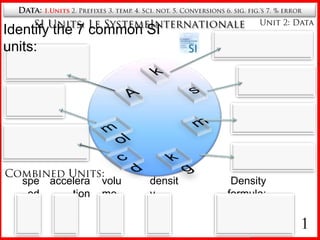
- 1. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error SI Units: Le SystemeInternationale Unit 2: Data Identify the 7 common SI units: Kelvin temperature Ampere k current second s time A mole meter amount mol m distance candela brightness cd kg kilogram mass Combined Units: speed acceleration density volume Density formula: 1
- 3. = (2 mL)(2.7 g/mL)
- 4. = 5.4 gdivide m multiply d v 2
- 5. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error Unit 2: Data Billion (109) Giga (G) 2. Prefixes Mega (M) Million (106) List the common unit prefixes and their abbreviations kilo (k) Thousand (103) learn more 1 Hundredth 10-2 centi (c) milli (m) Thousandth (10-3) micro (m) Millionth (10-6) 3 nano (n) Billionth (10-9)
- 6. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error Temperature Unit 2: Data When is Kelvin normally used and why? Kelvin Temp scale that cannot go below zero: K to 0C: 25oC = ?K K = oC +273 = 298 K In many formulas: it is mathematically accurate. 0F to 0C: °C= (°F - 32) × 5/9°F = ° C× 9/5 + 32 -40oC = ?0F x9 = -360, /5 = -72, + 32 = -40 0F -40oF = ?0C -32 = -72, x5 = -360, /9 = -40 = -40 0C 4 4
- 7. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error Scientific Notationfor big and small numbers Example: Try some: = 2.12 x 102 212 .0097 10,000 = 9.7x 10-3 = 1 x 104 602,000,000,000,000,000,000,000 Draw a line to make it between 1 and 10; count to decimal point. Always 10x Always 1-10 = 6.02x 1023 -2860 = -2.86 x 103 = 9.742 x 10-4 .0009742 5
- 8. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error Scientific Notation on your calculators: 2 methods Enter 6.02 x 1023 For fancy calculators (like TI-83, etc) For cheaper calculators that don’t do () Enter it all in parentheses- you’ll need them Use EE button, no parentheses needed Enter (6.02x10^23) Enter 6.02E23 Example: what is (2 x 101)(1 x 101)? Enter (2 x 10^1)(1 x 10^1) Enter 2E1x1E1 = 200 or 2E2 Try this: (3 x 10-2) x (-4.2 x 10-4) = ? Enter (3x10^-2)x(-4.2x10^-4) Enter 3E-2x-4.2E-4 Negative, not subtract Negative, not subtract Add answer = -1.26 x 10-5 or -0.0000126 6
- 9. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error 5. ConversionsA Sample Problem You have $7.25 in your pocket in quarters. How many quarters do you have? 1. Start with What you are given 3. Multiply using Conversion factors To get there 2. Write the units you need to Get to 4 quarters 29 = ___ quarters X 7.25 dollars dollar Cancel your units to prove you did it correctly. 7
- 11. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error 6. Significant Figures 1. When measuring include the known digits plus one estimated digit. Volume? 32.0 mL 9
- 12. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error Significant Figures: Guess which zero’s matter (L1 only) number why # SIG. FIGS: 1. “non-zero numbers are always significant” 32 2 2. “leading zeroes are Never significant” 0.0323 3 3. “sandwiched zeroes are always significant” 3.004 4 300 1 4. “trailing zeroes are only significant if there is a decimal place” 300. 3 5 300.20 .030690 Summary: Keep if decimal present dump 10 keep
- 13. Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error Learning Check INCLUDE add subtract multiply divide add graph to formula slide A. Which 2 answers contain 3 significant figures? 1) 0.4760 2) 0.00476 3) 4760 B. All the zeros are significant in 1) 0.00307 2) 25.300 3) 2.050 x 103 C. 534,675 rounded to 3 significant figures is 1) 535 2) 535,000 3) 5.35 x 105 > or = 5; round up 11
- 14. 7. Percent Error Data: 1.Units2. Prefixes 3. temp. 4. Sci. not. 5. Conversions 6. sig. fig.’s 7. % error You measure your mass to be 120 lbs, but in reality it is 150 lbs. What is your percent error? Add images, perhaps a video, etc. = 30 lbs/150 lbs x 100 = 20% End data unit 12