Mol.mass acids-bases
- 2. • Atom: The smallest particle of an element and that is chemically indivisible and can take part in chemical reaction. • Molecule: The smallest particle of element or compound that can exist freely. eg. H2, N2, H2O
- 3. Atomic mass an molecular mass mass of an atom • Atomic mass = --------------------- 1/12th mass of an atom of carbon12 Mass of a molecule • Molecular mass = ------------------------------------- 1/12th mass of an atom of carbon12
- 4. Molecular formula • A symbolic representation of molecule with the simplified ratio of the atoms present in it. • Significances of a molecular formula H2SO4 it contains …………of atoms of……… atom of ……………and ………………atoms of ………………. It indicates the number of different atoms present in it. It represent the correct proportion of each atom present in it. It helps to calculate the percentage of each element in it. It indicates the name. It helps to identify the raw materials required for its manufacture.
- 5. Mole • Mole: one gram molecular mass is called one mole. • Number of moles= mass/molecular mass • Calculation of molecular mass: • H2SO4 • Calculation of number of moles: how many moles are there in 36 g of water? • Given data: Mass of water=36 Molecular mass=18 no. of moles = 36/18 =2 moles Calculate the mass of 1.5 moles of ammonia?
- 6. Avogadro's hypothesis • "equal volumes of gases at the same temperature and pressure contain the same number of molecules regardless of their chemical nature
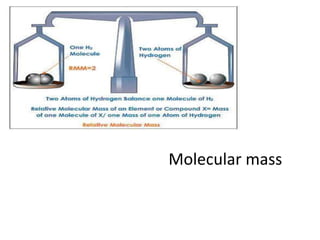
- 7. Relationship between molecular mass and vapour density • Vapour Density:It is the ratio of the mass of a volume of a gas, to the mass of an equal volume of hydrogen, measured under the same conditions of temperature and pressure. mass of certain volume of a gas • Vapour Density = --------------------------------------- Mass of same volume of H2 at the same P&T • According to Avogadro's law, equal volumes of all gases contain equal number of molecules.Thus, let the number of molecules in one volume = n, Therefore, Mass of ‘n’moleculesof a gas Vapour Density = -------------------------------------- Mass of n molecule of H2 • ‘n’can have any value; let n=1; • So, mass of a mlecule Vapour Density =-------------------------- Mass of H2 molecule • However, since hydrogen is diatomic., Mass of amolecule is nothing but Molecular mass ,Mass of H2 molecule is 2 • Molecular Mass = 2 x Vapour density •
- 12. Acids and bases • Which of the following are acids an bases identify them? • HCl, H2SO4, NaOH, Na2CO3, BF3, AlCl3, NH3, H2C2O4, • CH3COOH
- 15. Arrheneous Theory Arrhenius theory, introduced in 1887 by the Swedish scientist SvanteArrhenius, that acids are substances that dissociate in water to yield electrically charged atoms or molecules, called ions, one of which is a hydrogen ion (H +), and that bases ionize in water to yield hydroxide ions (OH −)
- 16. • The Bronsted-Lowry Theory of acids and bases. The theory. An acid is a proton (hydrogen ion) donor. A base is a proton (hydrogen ion) acceptor. • acid + base -> conjugate base + conjugate acid
- 17. In 1923 G. N. Lewis suggested • Lewis acid is anelectron-pair acceptor. A Lewis base is an electron- pair donor. • The Lewis acid-base theroy explains why BF3 reacts with ammonia. BF3 is a trigonal-planar molecule because electrons can be found in only three places in the valence shell of the boron atom. As a result, the boron atom is sp2 hybridized, which leaves an empty 2pzorbital on the boron atom. BF3 can therefore act as an electron-pair acceptor, or Lewis acid. It can use the empty 2pz orbital to pick up a pair of nonbonding electrons from a Lewis base to form a covalent bond. BF3 therefore reacts with Lewis bases such as NH3 to form acid-base complexes in which all of the atoms have a filled shell of valence electrons, as shown in the figure below. •
- 19. PHand pOH
- 20. Buffer • A buffer is a solution that can maintain a nearly constant pH if it is diluted, or if relatively small amounts of strong acids or bases are added. Buffer solutions resist pH changes. A buffer solution is typically made by mixing a weak acid and one of its salts OR mixing a weak base with one of its salts.
- 21. • Besides being toxic to cells, acidic blood also increases your risk of cancer, because it decreases oxygen and nutrient levels in your body; therefore, it can starve your cells of essential nutrients. Studies have shown that people with acidosis have higher risk of heart disease, cancer, osteoporosis, diabetes, arthritis and are more susceptible to diseases. • The human body works similar to an alkaline battery; thus, when its blood pH is too acidic, it does not function properly. As a result, the body’s energy decreases, cells cannot communicate properly, fungus and bacteria starts to grow out of control, and the body’s natural healing and defense system break down. The normal pH of human blood is slightly higher than 7.0. To be more specific, most experts agree that the normal pH of blood is between 7.35-7.45. If your blood pH is lower than 7.3, it is considered somewhat acidic.