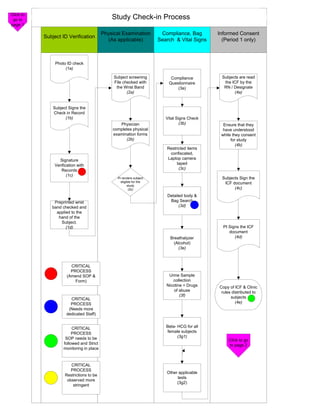
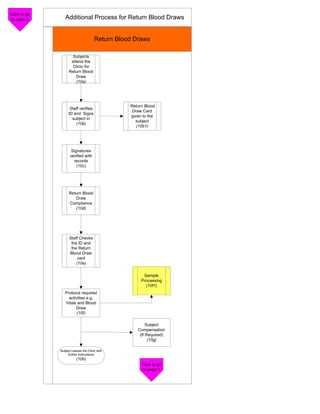
The document outlines the check-in and compliance processes for subjects participating in a study, including steps for physical examination, informed consent, and identification verification. It emphasizes the importance of detailed procedures and forms, as well as critical processes for monitoring and blood collection. Additionally, it highlights the need for staff dedication and stringent adherence to protocols throughout the study phases.