Embed presentation
Download to read offline

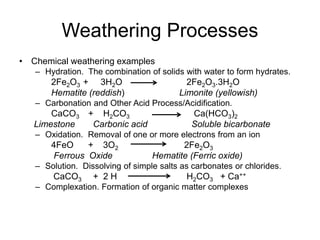

Chemical weathering breaks down the chemical structure and composition of minerals through various processes. These include hydrolysis where minerals react with water to form new minerals and acids, hydration where solids combine with water to form hydrates, carbonation where minerals react with carbon dioxide and acids to form soluble compounds, oxidation where electrons are removed from ions, and dissolution where simple salts dissolve. Factors that influence soil formation include climate, organisms, relief of the landscape, the parent material, and time, as described in models developed by Russian pedologist Dokuchaev and American soil scientist Jenny.


