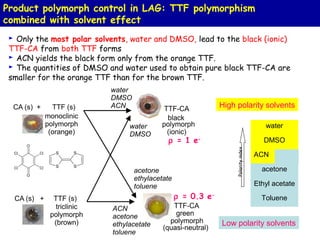
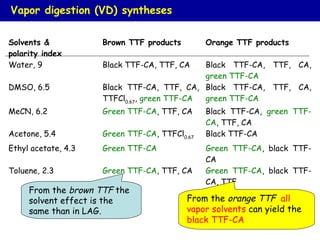
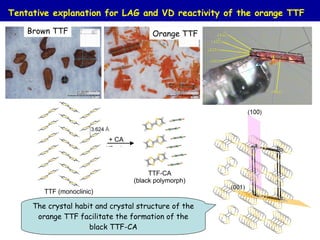
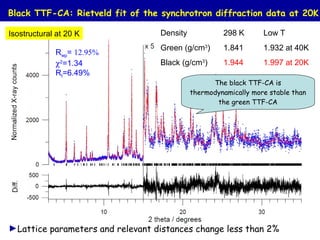
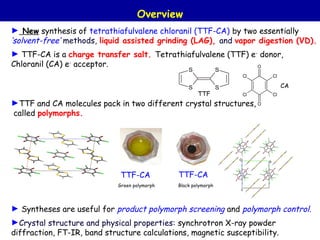
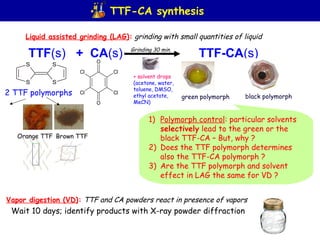
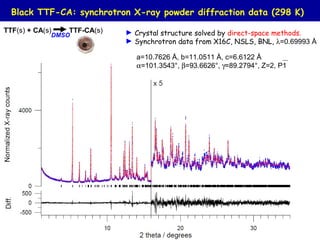
The document summarizes research on synthesizing and characterizing two polymorphs (crystal structures) of the charge transfer salt TTF-CA using solvent-free methods. The black polymorph was synthesized using liquid-assisted grinding and vapor digestion, with solvent polarity determining the resulting polymorph. Synchrotron X-ray diffraction was used to solve the crystal structure of the new black polymorph. Infrared spectroscopy determined the black polymorph is a semiconductor with a band gap of 0.198 eV that remains ionic from 10-300K.

![1 0 1 5 2 0 2 5 3 0 3 5 4 0 4 5 5 0 5 5
2 t h e t a / d e g r e e s
0
2 0 0 0 0
4 0 0 0 0
6 0 0 0 0
X-raycounts
- 3 0 0 0
0
3 0 0 0
Difference
acetone
TTF(s) + CA(s) TTF-CA(s) [1]
[1]- S. Benjamin et al. Synthetic Metals 161 (2011) 996-1000; [2]- Mayerle et al, Acta Cryst. (1979) B35, 2988-2995.
Green TTF-CA: Rietveld fit of the laboratory XRPD data
► Crystal structure known (1979). [2]
► Neutral (ρ=0.3e-
) to ionic (0.7e-
)
transition at 81K and 1atm, together with a
crystallographic phase transition (P21/n to Pn)
Viewed along [010] (T=300K)
ρ=0.3e-
(pseudo-neutral)](https://image.slidesharecdn.com/d76c39a5-fa6f-4425-a1fa-6ef0e48b27cd-160428234643/85/VAS-May-2014-Pagola-current-4-320.jpg)
![Black TTF-CA: crystal structure analysis
►Experimentally DIAMAGNETIC (magnetic susceptibility balance)
Viewed along [001]: eclipsed
(TTF+●
)2
radical cation dimers;
typical packing motif of ionic,
diamagnetic and insulating TTF compounds
Viewed along [110]: columns of TTF
and (CA-●
)2 radical anion dimers](https://image.slidesharecdn.com/d76c39a5-fa6f-4425-a1fa-6ef0e48b27cd-160428234643/85/VAS-May-2014-Pagola-current-6-320.jpg)