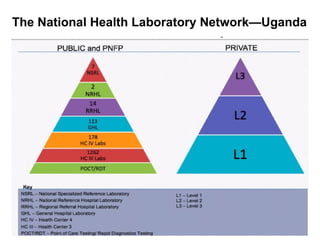
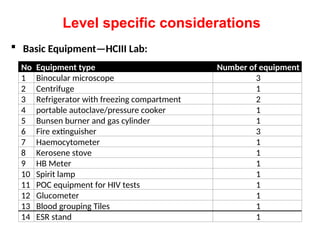
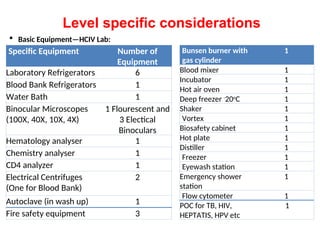
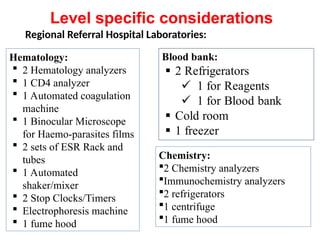
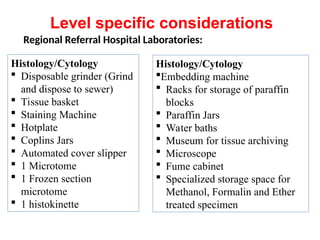
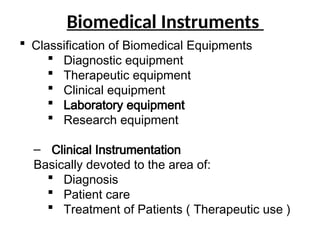
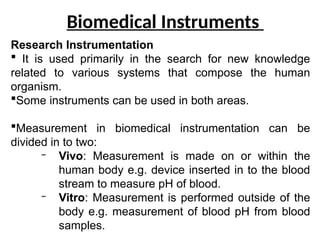
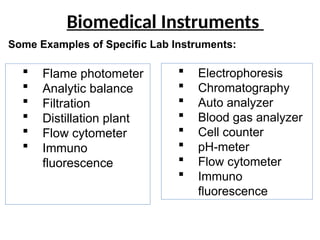
The document outlines key equipment requirements for various levels of health laboratories in Uganda, detailing specific needs for health center III, IV, general hospitals, and regional referral hospitals. Each section lists essential equipment types and quantities necessary to perform various diagnostic tests while adhering to specified biosafety levels. It also highlights the importance of proper laboratory management, equipment selection, and maintenance for effective healthcare service delivery.