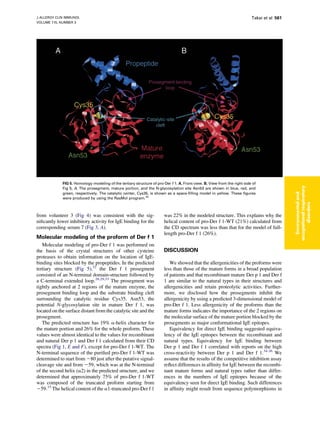
This study investigated the allergenic properties of recombinant pro- and mature forms of the major house dust mite allergens Der p 1 and Der f 1. The recombinant mature forms exhibited similar structure and allergenicity as the natural allergens. However, the recombinant proforms had different structures than the mature forms and showed reduced allergenicity in IgE binding and histamine release assays using patient sera. Molecular modeling suggested the prodomains block major IgE epitopes on the mature allergen surfaces. This indicates the prodomains reduce allergenicity by masking key epitopes.