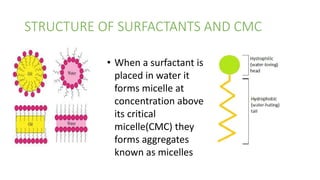
Surfactants are amphiphilic molecules that reduce the surface tension of water. They were first observed by J.W. Gibbs to concentrate at liquid surfaces more than in bulk solutions, as expressed by Gibbs' adsorption equation. Surfactants form micelles, spherical aggregates of surfactant molecules, above their critical micelle concentration in water. Examples of surfactants include stearic acid.