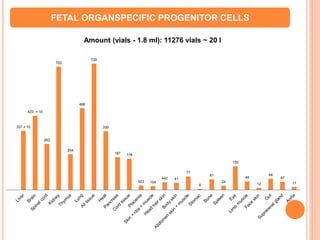
1. The document describes the facilities and procedures of Cryobank at EmProCell Clinical Research Pvt. Ltd. in Mumbai for processing and storing fetal tissue extracts and organ-specific progenitor cells.
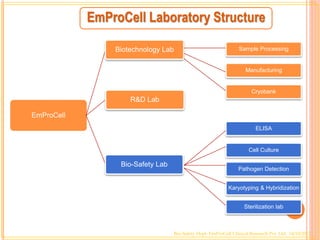
2. EmProCell has laboratories for biotechnology, biosafety, research and development, sample processing, manufacturing, cryobanking, ELISA testing, cell culture, pathogen detection, karyotyping and hybridization, and sterilization.
3. Donor screening involves ELISA testing of pregnant women's blood for HBV, HCV, CMV, HTLV, and VDRL. Samples then undergo pathogen detection by PCR and sterility testing before being processed and cryop











![PASSPORT OF CELL SUSPENSION
Sample ID: (_ _._ _._ _/_)(_)(_ _ _)(_ _ _ _ _ _)
Type of testing Marker Result Marker Result
Screening Donors
(ELISA)
HIV 1 [ ] CMV [ ]
HIV 2 [ ] HTLV [ ]
HBV [ ] VDRL [ ]
HCV [ ]
Cell test-control
(PCR)
Pathogen Result Pathogen Result
HBV [ ] HCV [ ]
CMV [ ] EBV [ ]
HSV 1 & 2 [ ] HIV 1 & 2 [ ]
Treponema pallidum [ ] Mycoplasma hominis [ ]
Ureaplasma sp. [ ] Chlamydia (all species) [ ]
Toxoplasma gondii [ ] X-Y chromosome detection [ ]
Microbiology control
Sterility testing
Pathogen Result Pathogen Result
Aerobic m/o [ ] Fungus [ ]
Anaerobic m/o [ ]](https://image.slidesharecdn.com/cryobank-151121144916-lva1-app6891/85/Sunil-Saldanha-14-320.jpg)
![PASSPORT OF CELL SUSPENSION
Gestation period of the
human fetus
(mark as [√])
Weeks Result Weeks Result Weeks Result
6-7 weeks [ ] 13 weeks [ ] 17 weeks [ ]
8-9 weeks [ ] 14 weeks [ ] 18 weeks [ ]
10-11 weeks [ ] 15 weeks [ ] 19 weeks [ ]
12 weeks [ ] 16 weeks [ ] 20 weeks [ ]
Cell type
(mark as [√])
Hematopoietic fetal liver stem cells [ ]
Fetal liver hepatoblasts and hepatocytes [ ]
Neural progenitor fetal brain cells [ ]
Quantity & Quality Total Cell Amount per 1 ml
Vible-Cell Amount per 1 ml
Amount of CFU per 1 ml
Amount of mononuclear cells
Karyotyping (FISH)
Transplantation unit: amount of CFU/amount of viable cells
Cryo-protector (mark as
[√])
DMSO [ ]
PVG [ ]
Any other (Name)](https://image.slidesharecdn.com/cryobank-151121144916-lva1-app6891/85/Sunil-Saldanha-15-320.jpg)
![PASSPORT OF CELL SUSPENSION
Phenotype of fetal liver hematopoietic stem cells
HLA typing (Type)
CD Marker
(mark as [√])
CD Marker Result CD Marker Result CD Marker Result
CD4 [ ] CD56 [ ] CD133 [ ]
CD8 [ ] CD62E [ ] CD135 [ ]
CD12w [ ] CD62P [ ] CD141 [ ]
CD19 [ ] CD69 [ ] CD144 [ ]
CD24 [ ] CD74 [ ] CD158 [ ]
CD27 [ ] CD83 [ ] CD163 [ ]
CD31 [ ] CD90 [ ] CD184 [ ]
CD34 [ ] CD100 [ ] CD202a [ ]
CD42 [ ] CD105 [ ] CD326 [ ]
CD45 [ ] CD117 [ ] CD133 [ ]
Marker Phenotype of fetal liver hepatoblasts and hepatocytes
Result Marker Result Marker Result
AFP [ ] HLA-A [ ] CD74 [ ]
CD326 [ ] HLA-B [ ] CD135 [ ]
Albumin [ ] HLA-DR [ ] CD326 [ ]
C017-1A antigen [ ] CD68 [ ]](https://image.slidesharecdn.com/cryobank-151121144916-lva1-app6891/85/Sunil-Saldanha-16-320.jpg)
![PASSPORT OF CELL SUSPENSION
Phenotype of neural fetal brain progenitor cells
Marker Result Marker Result Marker Result
Nestin [ ] HLA-A [ ] CD133 [ ]
β-tubulin III [ ] HLA-B [ ] CD271 [ ]
Vimentin [ ] HLA-DR [ ] CD304 [ ]
GFAP [ ] CD24 [ ] CD326 [ ]
NCAM [ ] CD25 [ ] CD69 [ ]
GalC [ ] CD74 [ ]
Genetic abnormality in cells if any detected by karyotyping:
Please enlist _____________________________________________________________________
_______________________________________________________________________________
_________________________________________________________________________________________________________________
_____________________________________________](https://image.slidesharecdn.com/cryobank-151121144916-lva1-app6891/85/Sunil-Saldanha-17-320.jpg)
