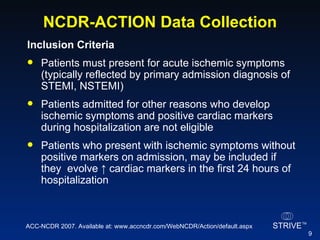
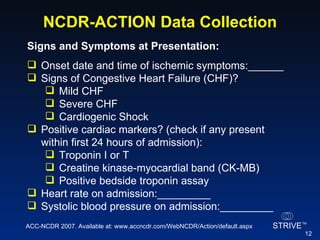
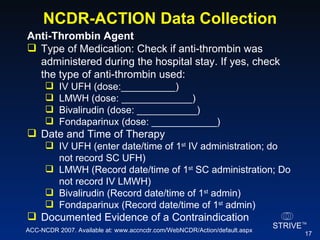
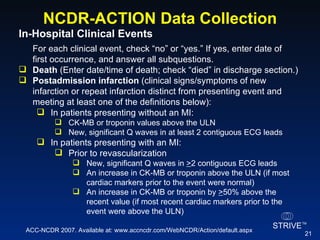
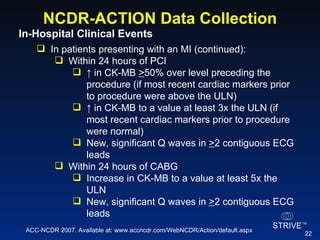
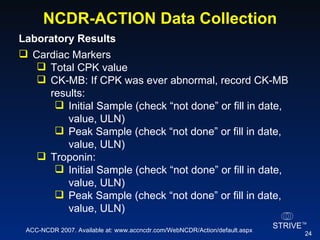
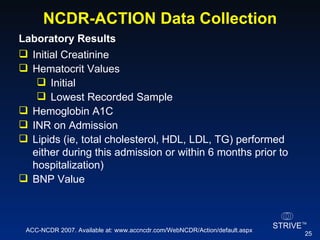
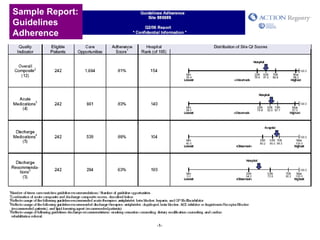
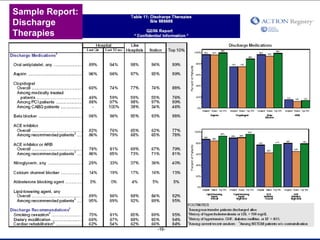
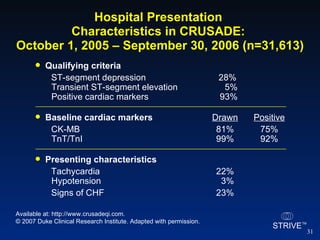
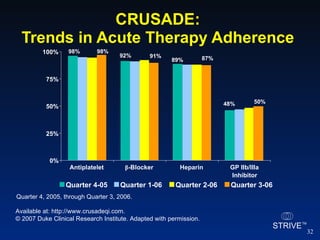
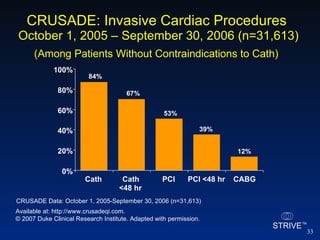
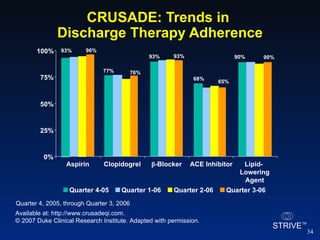
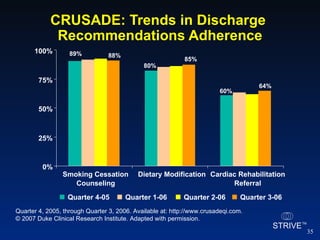
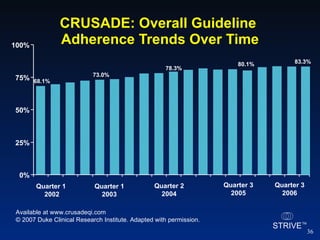
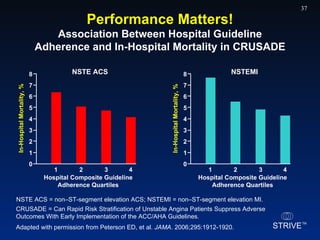
The document summarizes an educational activity on the NCDR-ACTION Registry, which combines data from the CRUSADE and NRMI registries on the treatment of acute coronary syndromes. It describes the features and data collection process for the NCDR-ACTION Registry, including patient presentation characteristics, medical history, in-hospital treatments, outcomes, and sample institutional reports. The faculty disclosure indicates relationships with pharmaceutical companies supporting the activity.