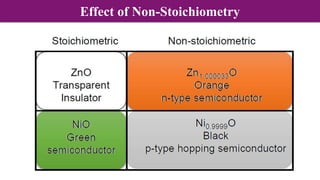
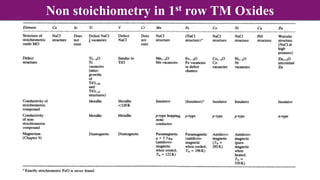
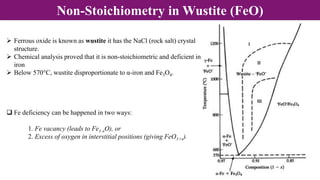
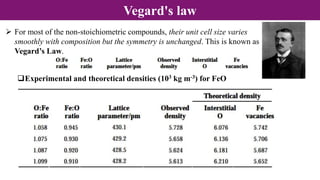
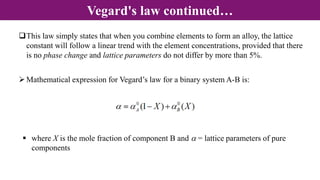
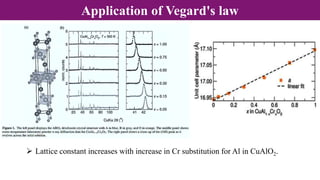
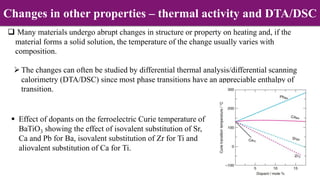
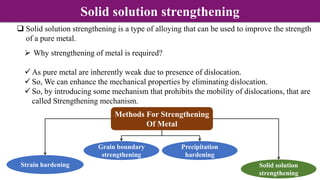
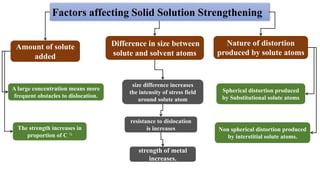
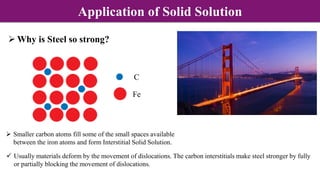
Non-Stoichiometry & Solid Solution
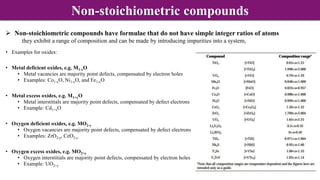
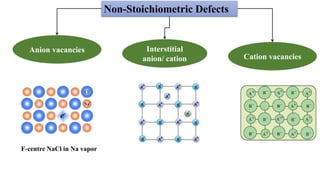
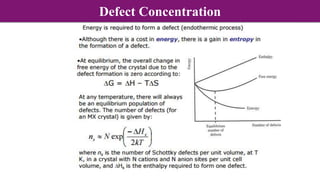
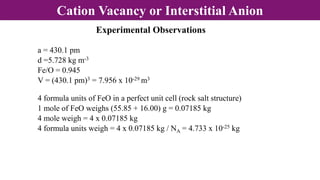
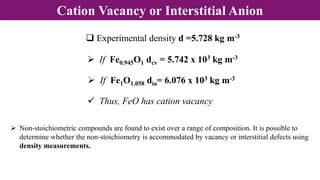
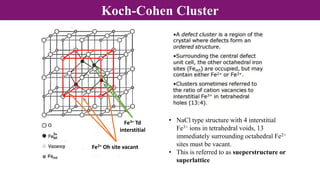
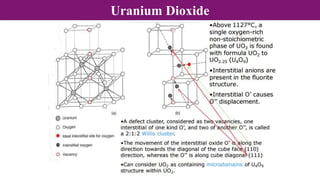
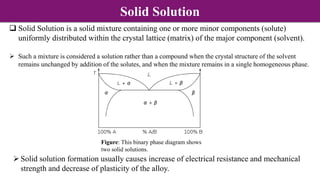
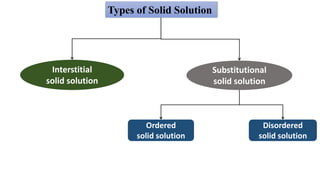
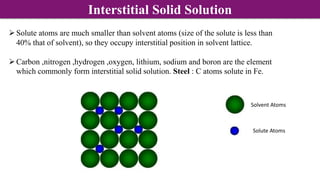
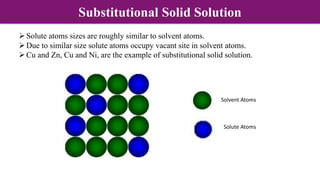
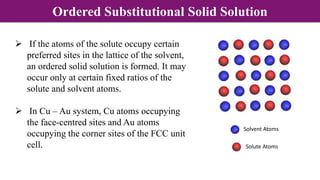
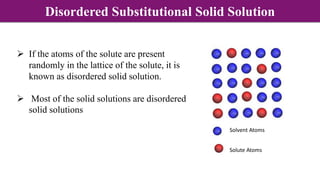
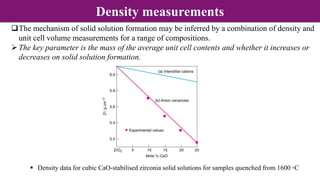
The document discusses various types of point defects that can occur in non-stoichiometric compounds including Schottky defects, Frenkel defects, and anti-site defects. It provides examples of intrinsic point defects in metal-deficient, metal-excess, oxygen-deficient, and oxygen-excess metal oxides. Solid solutions are formed when one or more minor components dissolve uniformly within the crystal lattice of a major component. Types of solid solutions include interstitial, substitutional, ordered, and disordered solutions. Experimental techniques like X-ray diffraction and density measurements can be used to study properties of non-stoichiometric compounds and solid solutions.