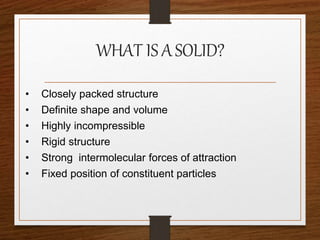
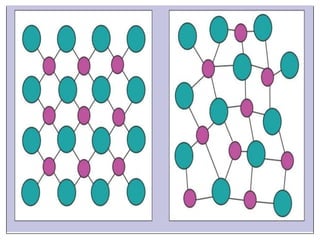
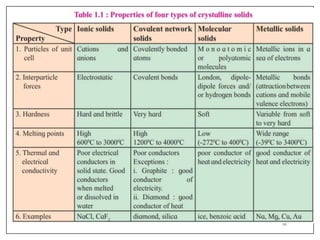
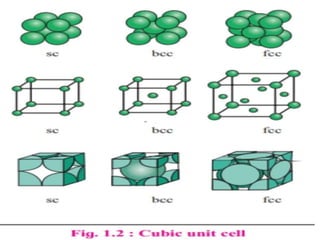
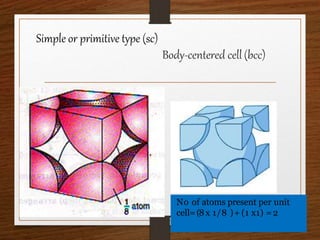
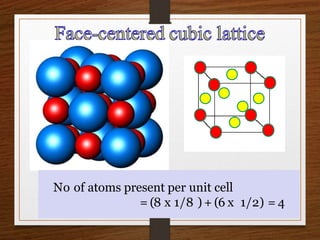
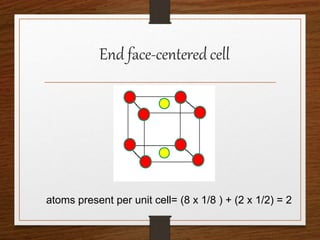
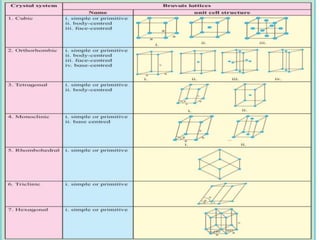
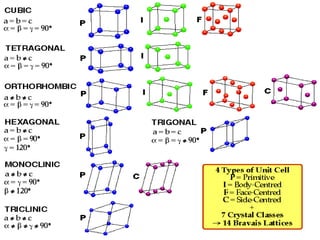
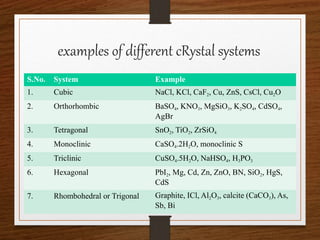
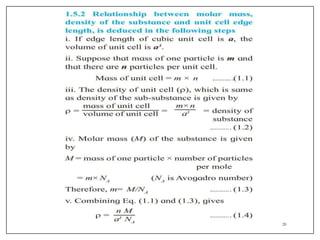
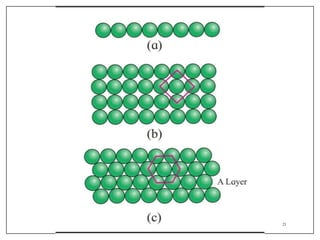
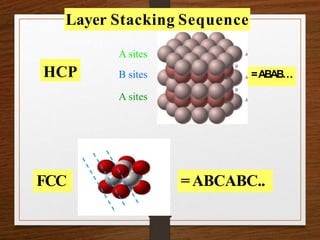
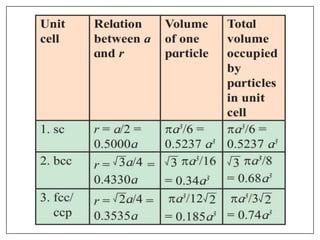
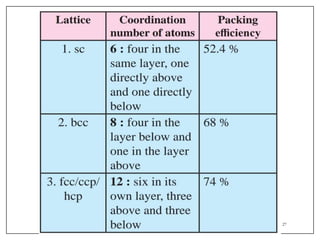
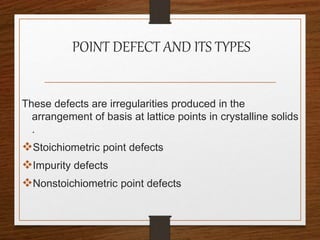
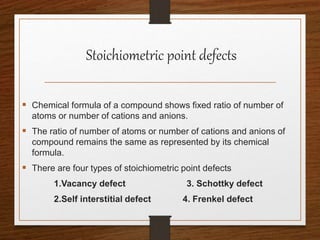
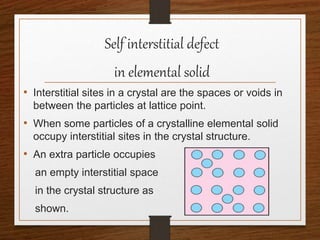
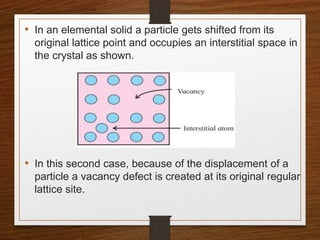
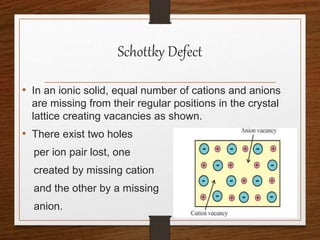
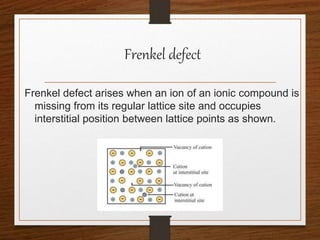
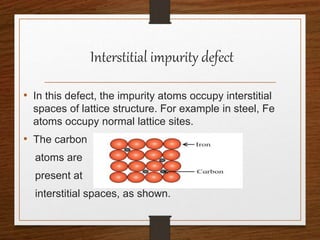
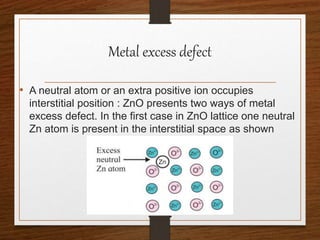
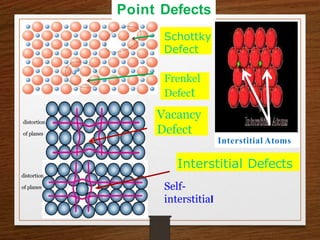
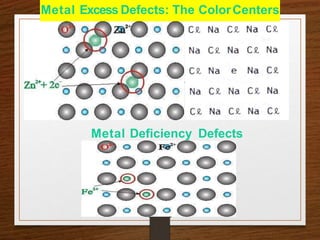
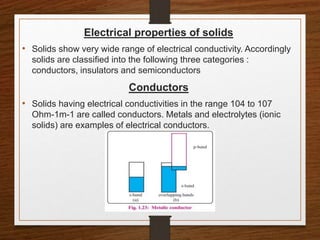
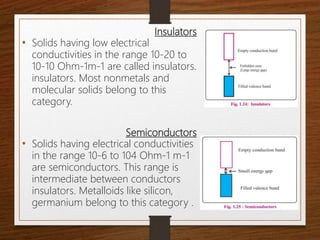
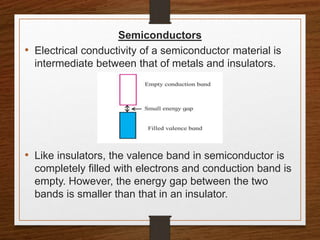
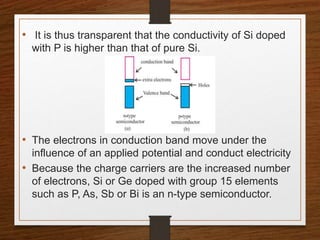
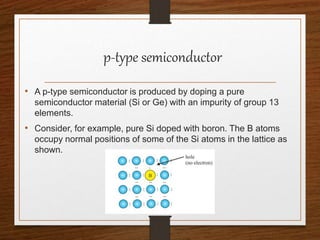
This document provides an overview of solid state properties. It defines solids as having a definite shape and volume, and being highly incompressible due to strong intermolecular forces. Solids are classified as crystalline, polycrystalline, or amorphous based on atomic arrangement. Crystalline solids have a regular atomic arrangement while amorphous solids lack order. Different types of crystal structures and defects are also discussed.