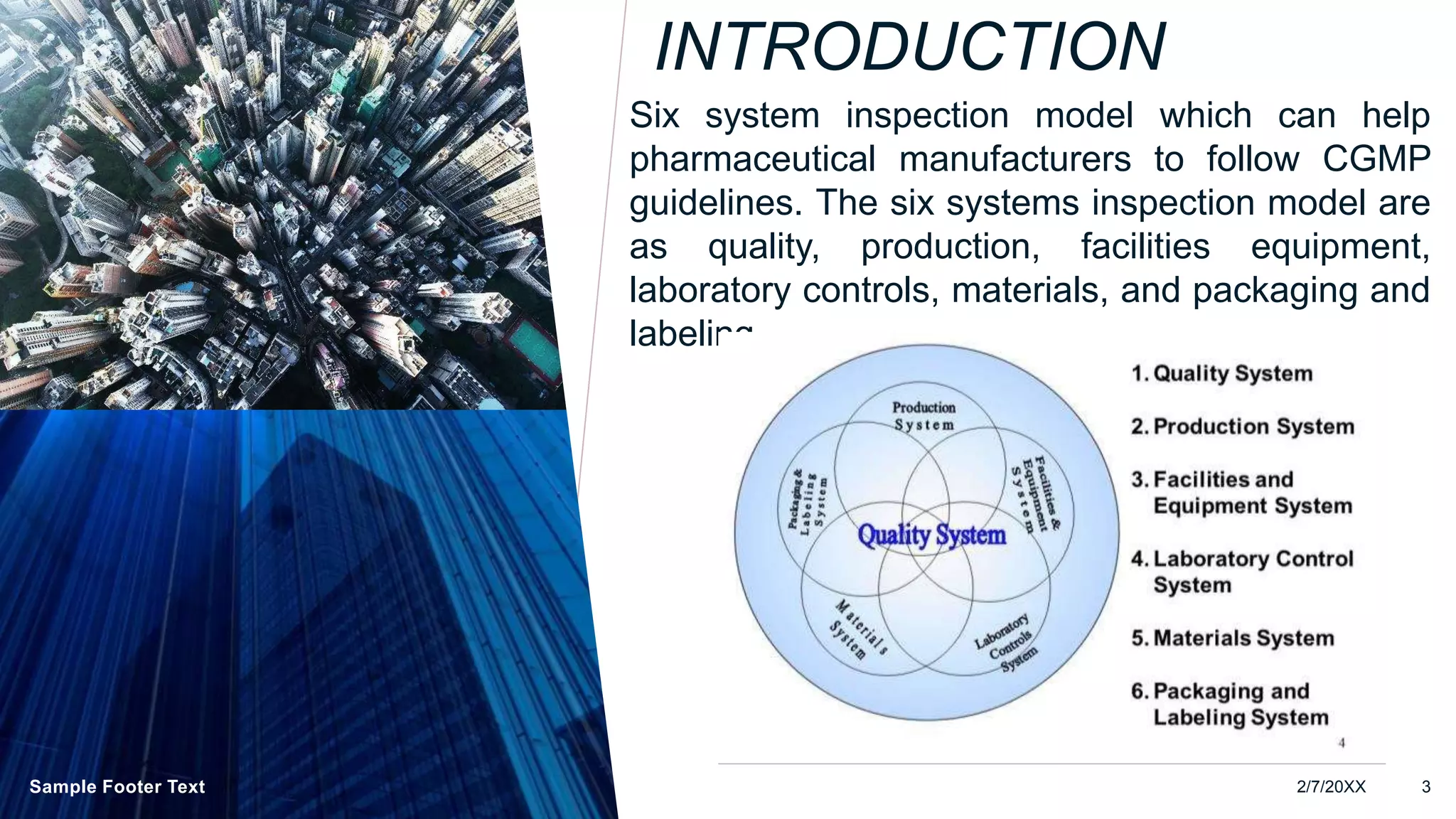
The document discusses the Six System Inspection Model which can help pharmaceutical manufacturers follow CGMP guidelines. The six systems are: quality management, facility and equipment, laboratory controls, materials, packaging and labeling, and change management. Each system ensures different aspects of compliance such as quality planning, production processes, equipment qualification, testing, materials control, and packaging and labeling validation. Self-inspection and change control are also important concepts that are reviewed.