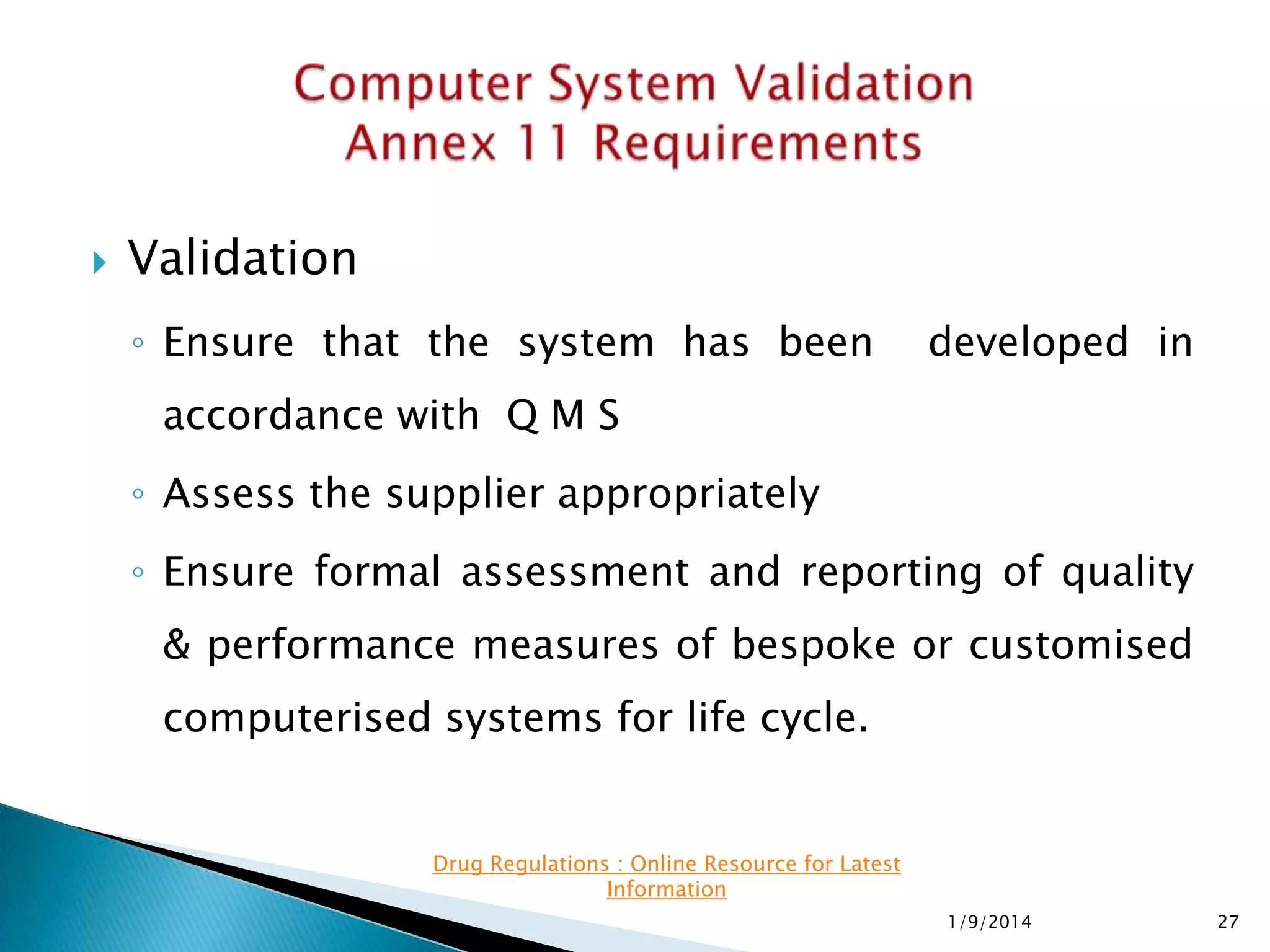
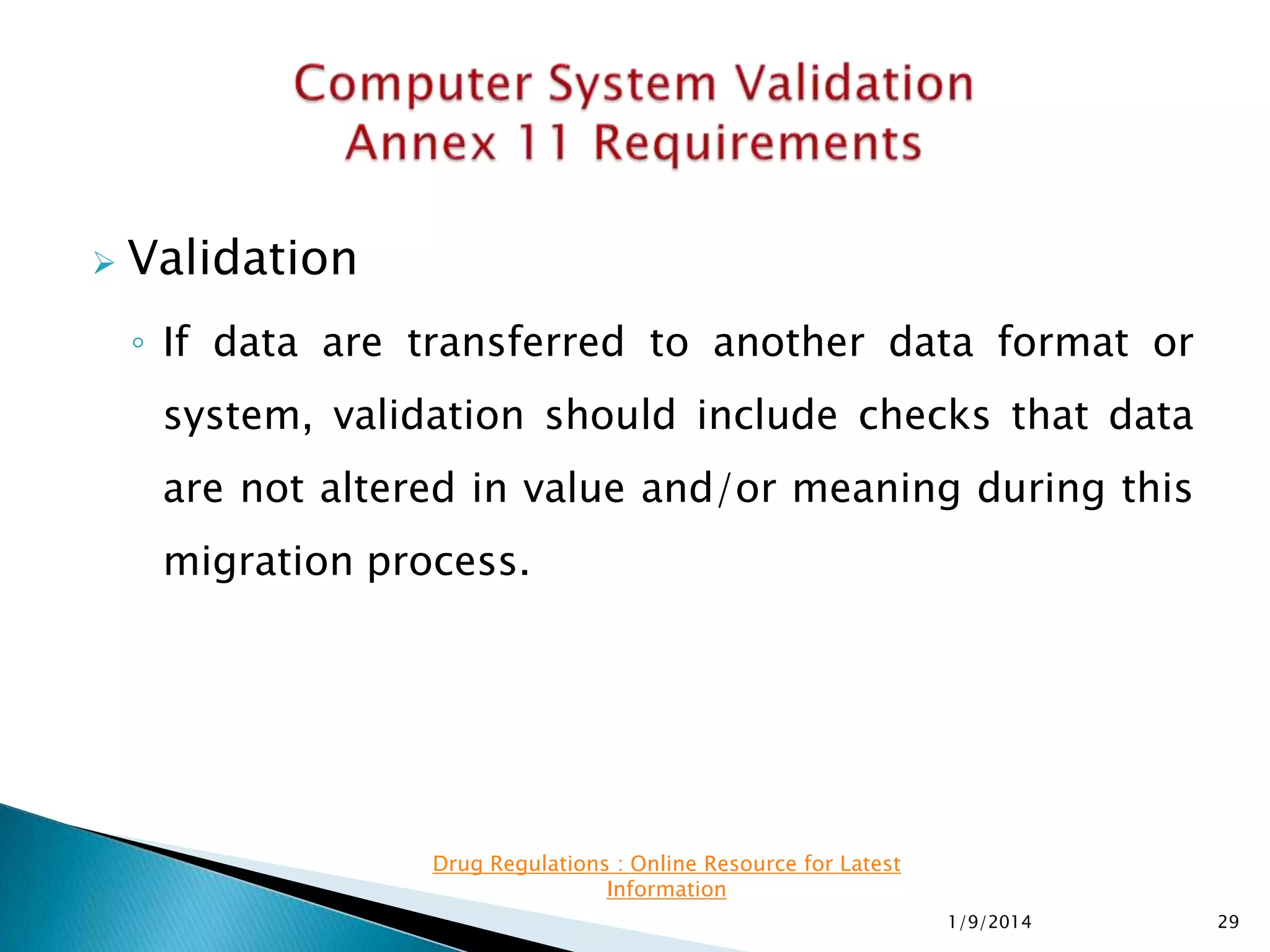
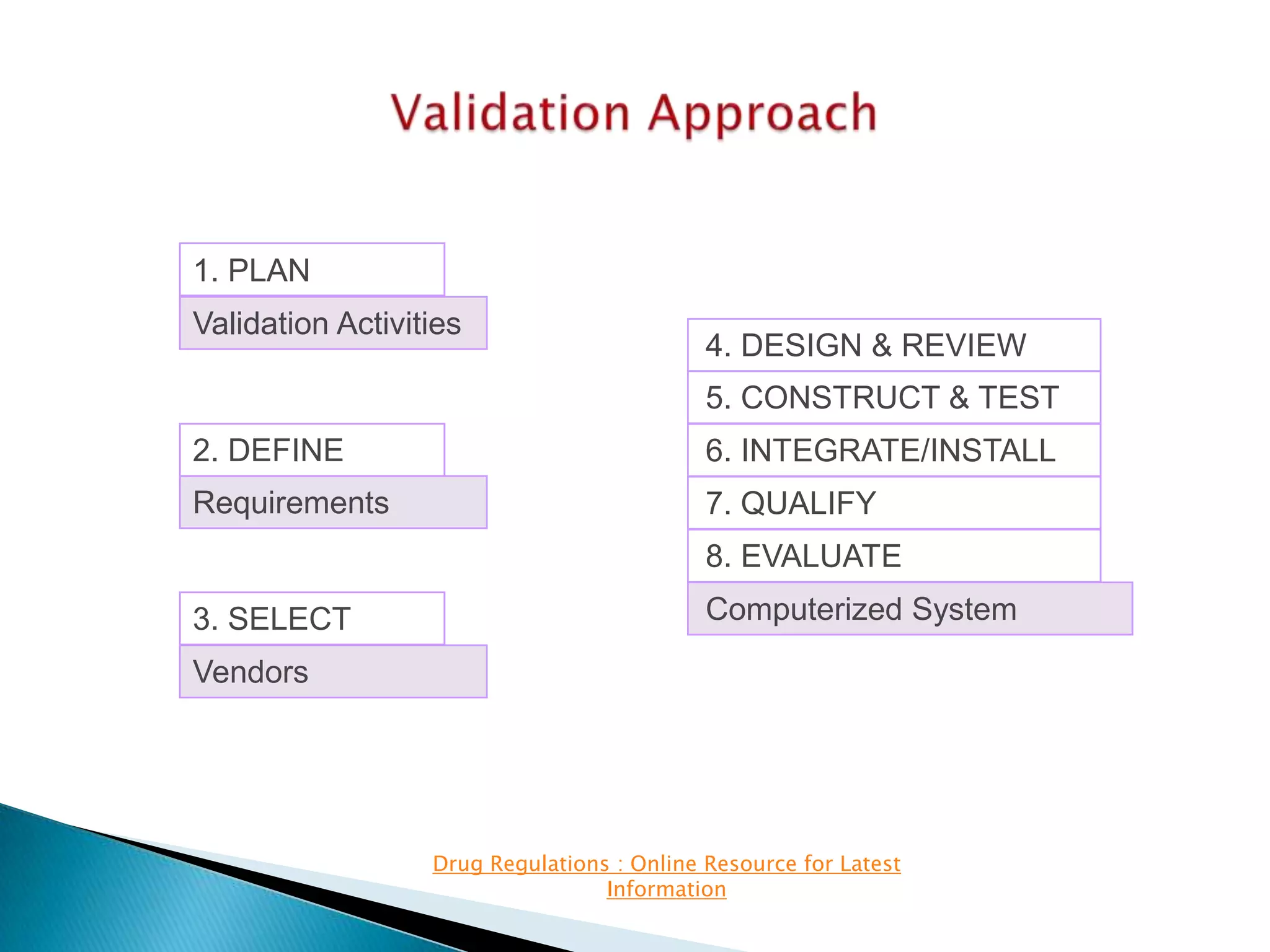
The presentation details software validation approaches for the pharmaceutical industry, highlighting the importance of compliance with regulatory requirements, particularly with regard to computerized systems. It emphasizes the collaborative roles of users, developers, and management in ensuring proper validation processes, while also discussing risks associated with system failures and the need for thorough documentation. The FDA's guidance on validation and enforcement discretion for legacy systems is also reviewed, outlining key responsibilities and expectations throughout the system lifecycle.