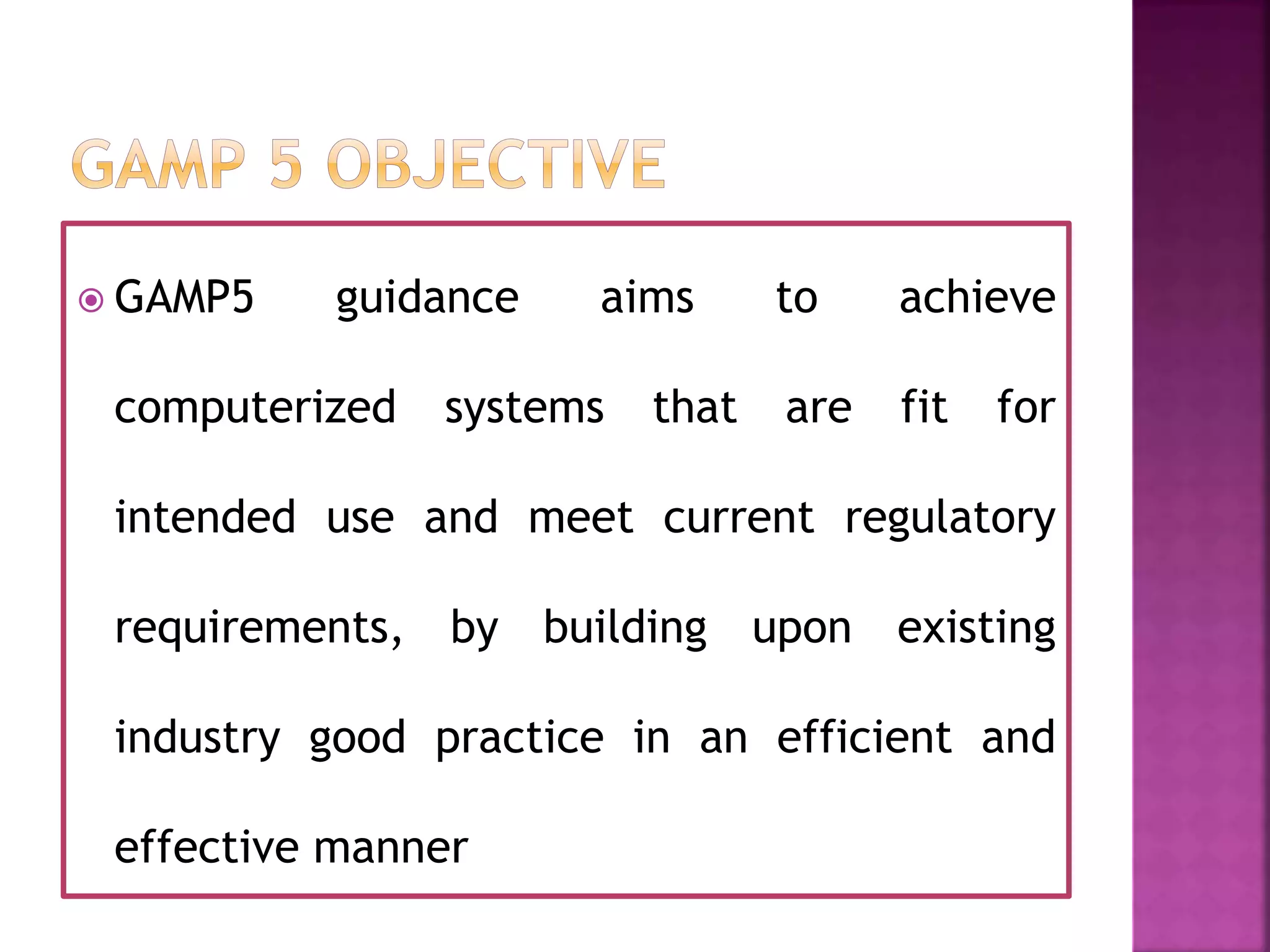
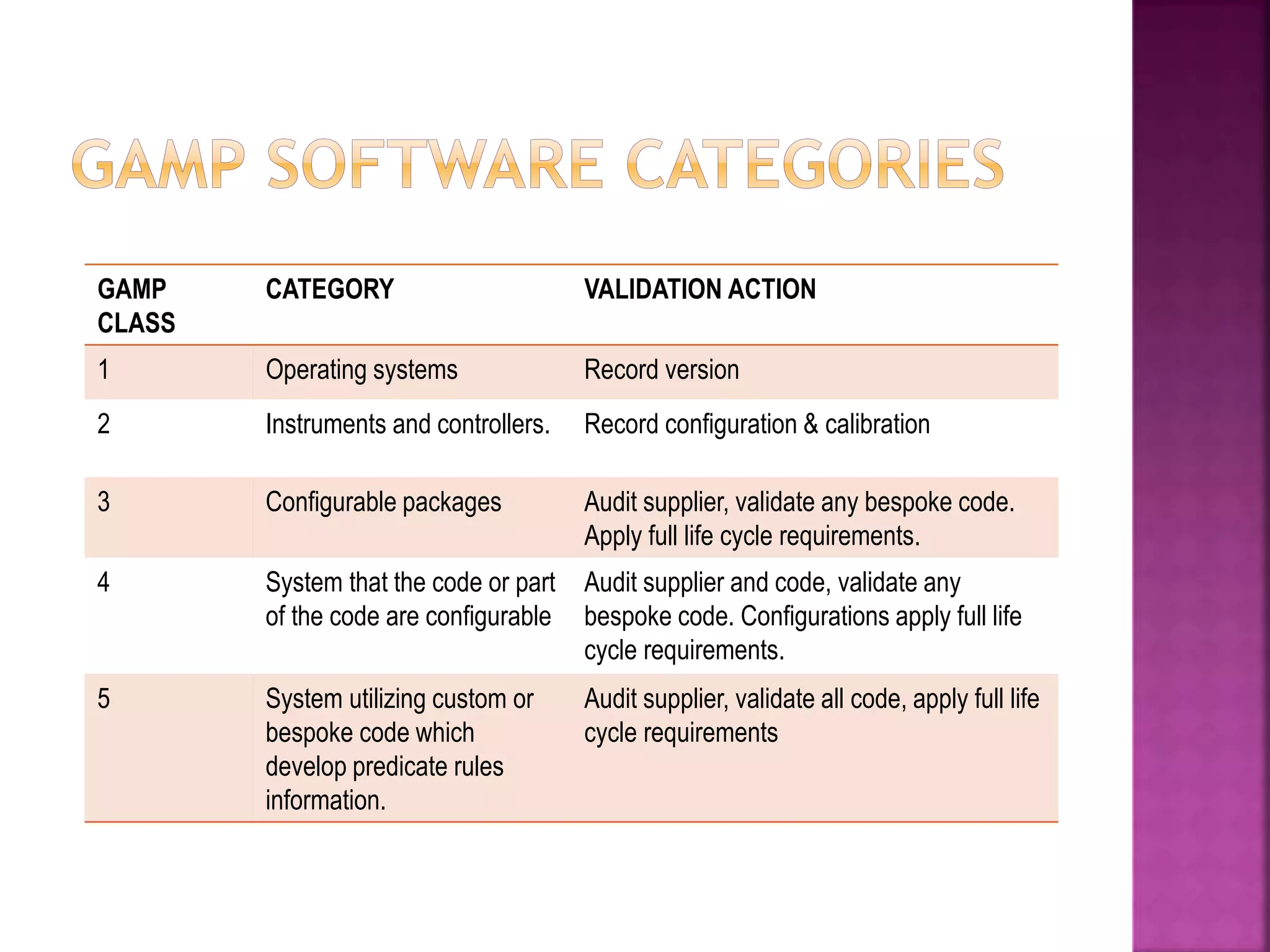
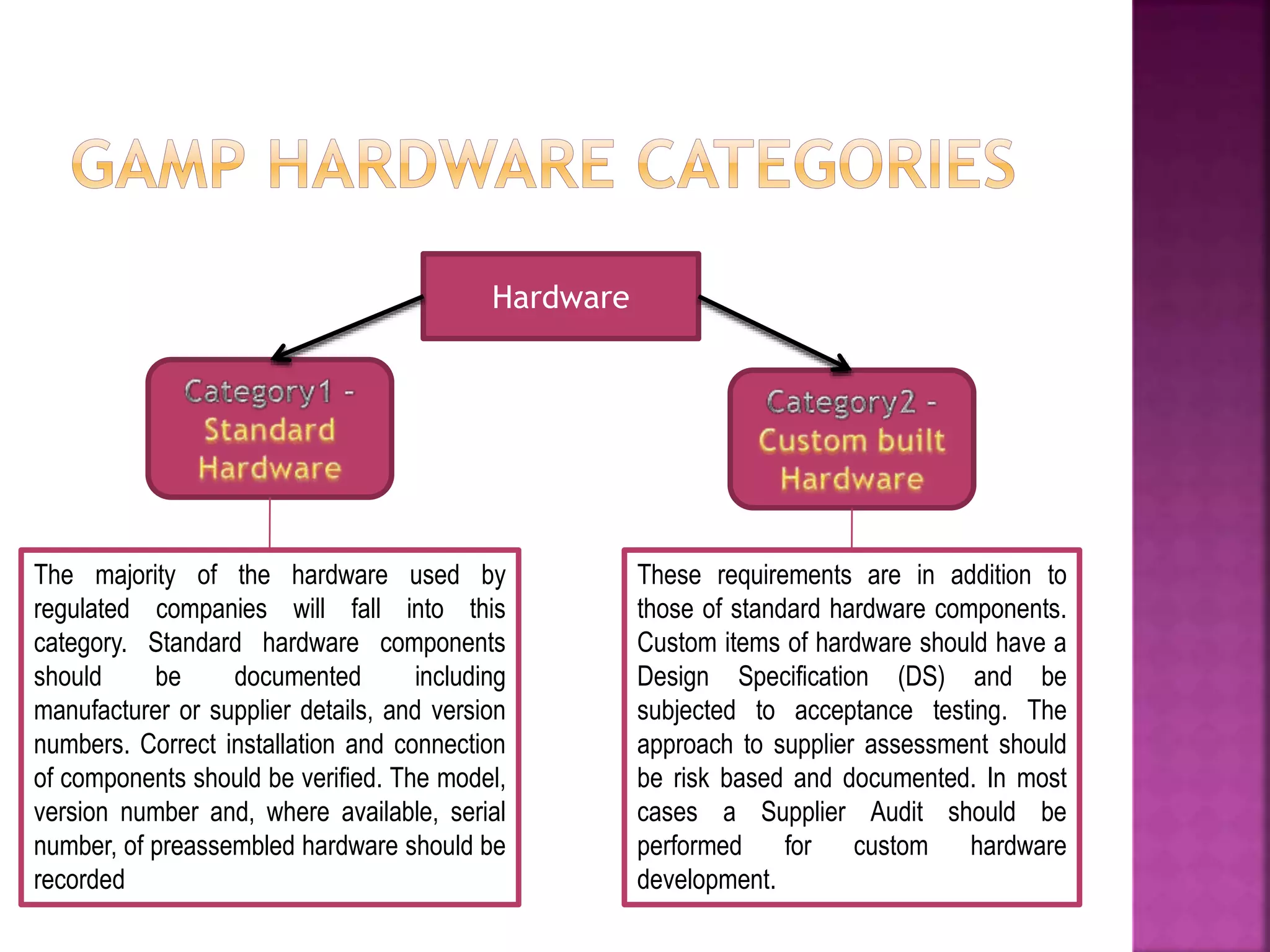
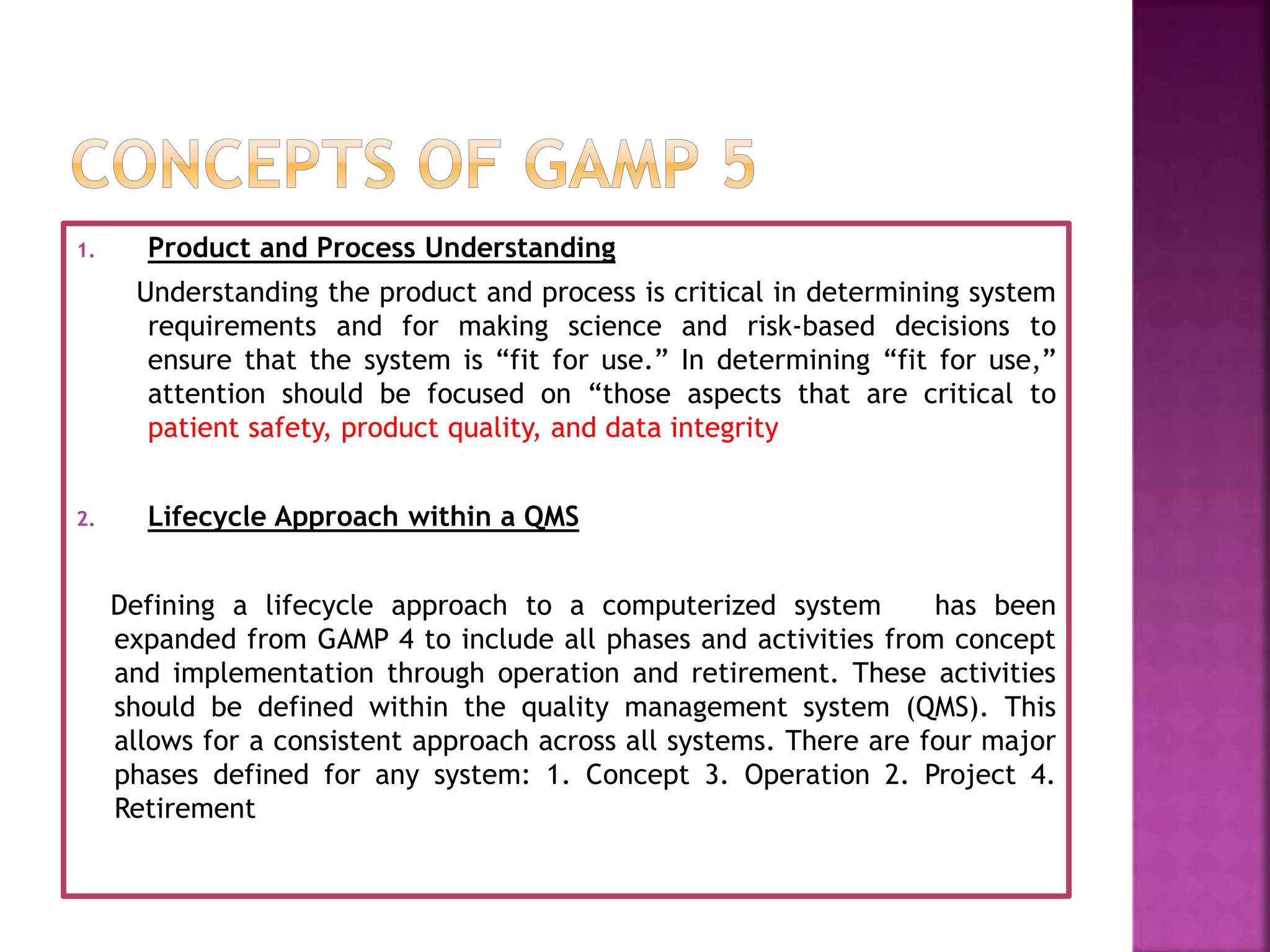
The document discusses Good Automated Manufacturing Practice (GAMP), which are guidelines for manufacturers and users of automated systems in the pharmaceutical industry published by the International Society for Pharmaceutical Engineering (ISPE). GAMP aims to ensure pharmaceutical products have the required quality by establishing principles and procedures for validating automated systems. Key aspects of GAMP covered in the document include focusing on building quality into each stage of manufacturing rather than testing it in, covering all production aspects from raw materials to staff training. The document also summarizes the GAMP5 guidelines released in 2008, which provide a framework for validating computerized systems to ensure they are fit for use and compliant with regulations. GAMP5 emphasizes product and process understanding, a lifecycle approach,






![ P. Lalasa & Vishal Gupta et.al., A Review on applications of GAMP – 5 in
Pharmaceutical Industry, Jss university, July- September 2013, Vol. 5, Issue 3, ISSN
0975 – 9344.
GAMP®5: A Risk-Based Approach to Compliant GMP Computerized Systems. ©
Copyright ISPE 2008. All right reserved. [Cited 2012 Dec 26].Available from:
www.ispe.org/publications/gamp4togamp5.pdf
ISPE GAMP-5 A Risk-Based Approach to Compliant GMP Computerized Systems,
International society for pharmaceutical engineering (ISPE), Fifth edition,
Febrauary-2008, – applying management based on the business process. [Cited 2012
Dec 26]. Available from: www.techstreet.com/products/preview/1559506
Application Description AD/Randi/005-EN.
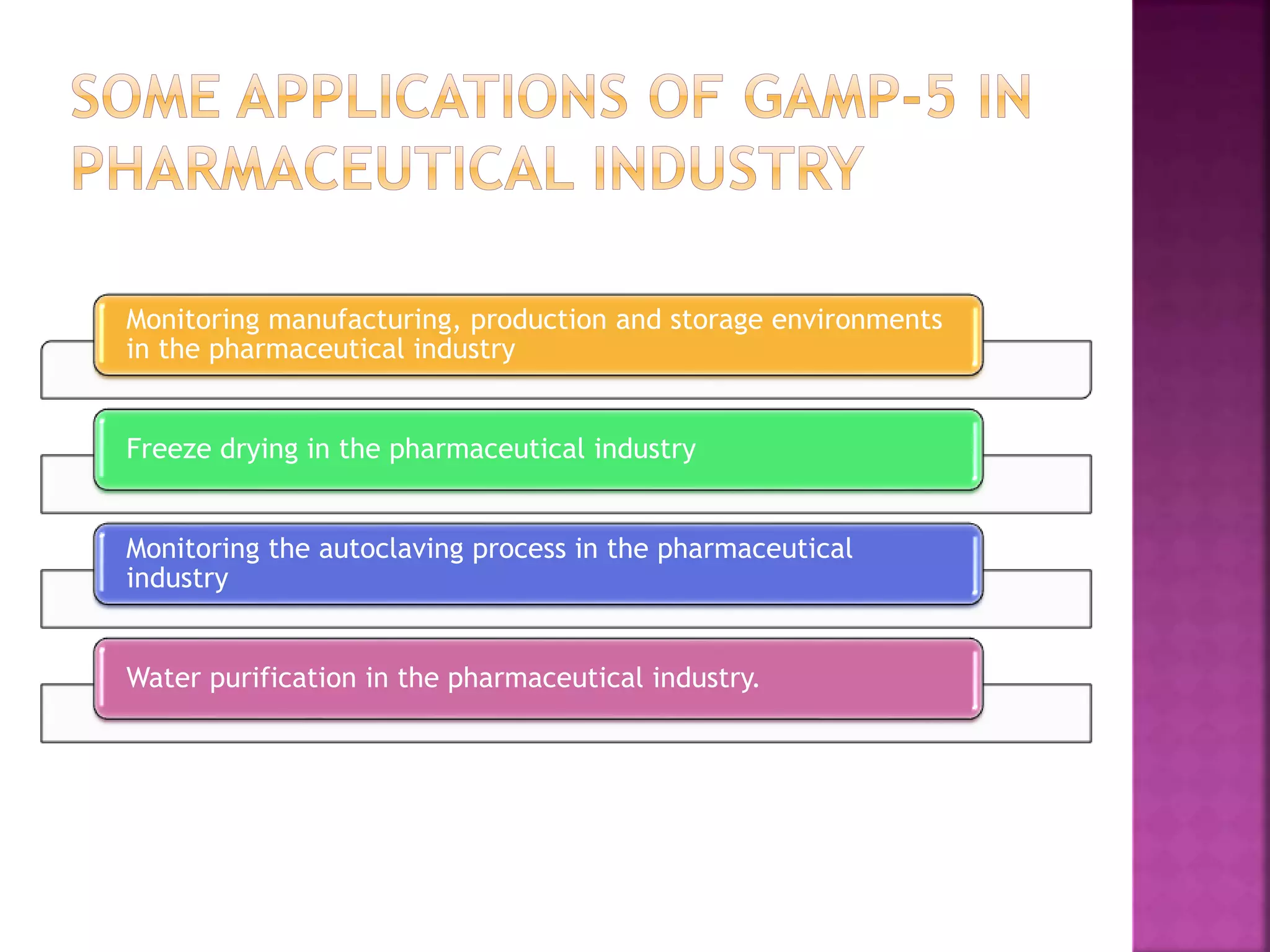
Monitoring manufacturing, production and storage environments in the
pharmaceutical Industry. [Cited 2012 Dec 26]. Available from:
http://www05.abb.com/global/scot/scot203.nsf/veritydisplay/e1a32babfc8ddeb1](https://image.slidesharecdn.com/gamp-160509100148/75/Good-Automated-Manufacturing-Practices-19-2048.jpg)
