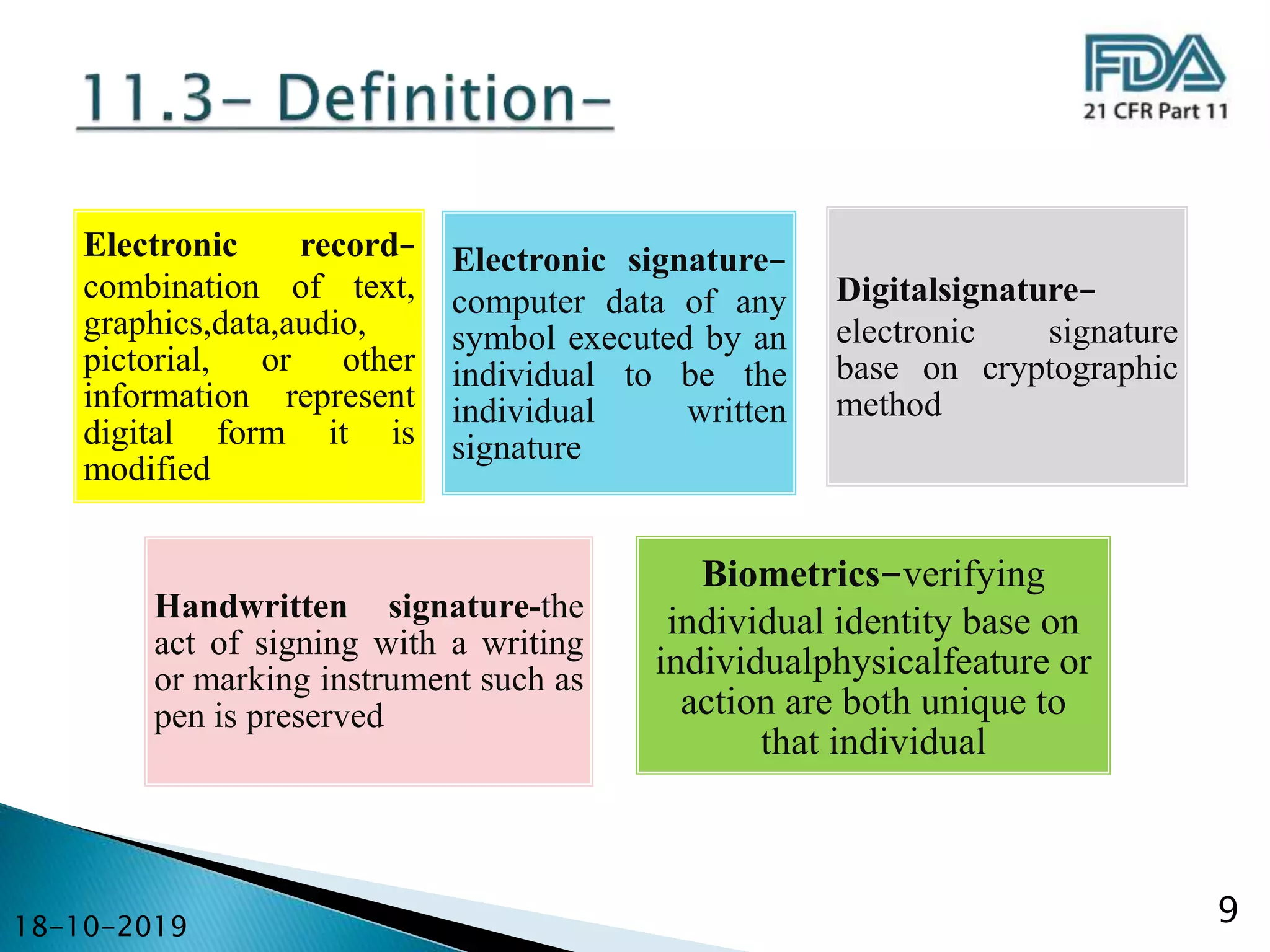
This document provides an overview of 21 CFR Part 11, which establishes criteria for electronic records and electronic signatures. It discusses how Part 11 falls under Title 21 of the Code of Federal Regulations, which regulates the food and drug industry. The document then examines the key aspects of Part 11, including its scope, definitions of electronic records and signatures, requirements for controls on closed and open systems, requirements for electronic signature components and security, and general record keeping requirements. It provides examples of how these regulations are implemented in pharmaceutical organizations and clinical trials.