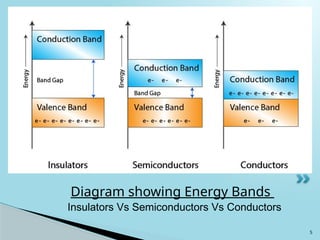
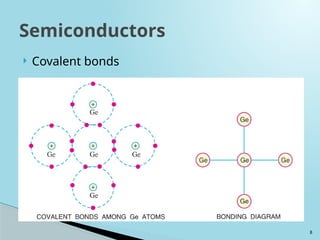
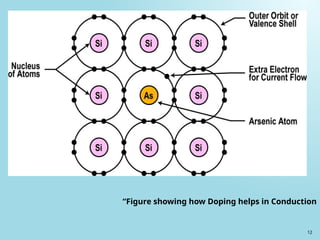
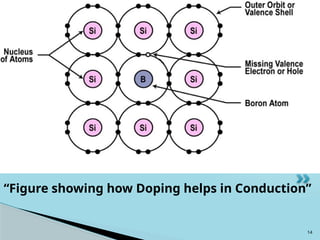
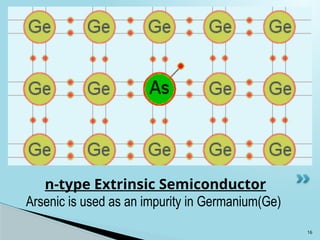
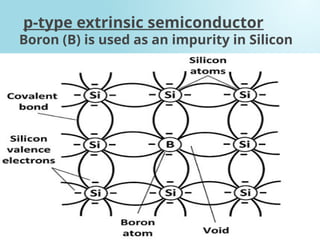
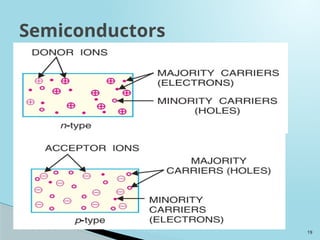
Semiconductors are materials with electrical conductivity between conductors and insulators, crucial for modern electronics. Key semiconductor materials include silicon, germanium, and gallium arsenide, and their properties can be modified through doping. These materials enable various applications such as diodes, transistors, and sensors, making them essential for technological advancement and economic growth.