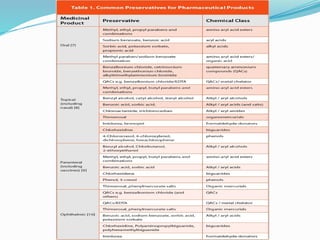
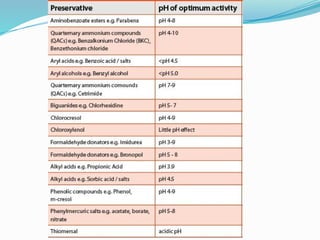
This document defines preservatives and discusses their use and classification. Preservatives are added to foods and pharmaceuticals to prolong shelf life by preventing microbial growth. They are classified as antimicrobials, which kill microbes, or antioxidants, which prevent oxidation. Important criteria for selecting preservatives include effectiveness against microbes, stability, nontoxicity, and compatibility. Common antimicrobial types are alcohols, acids like benzoic acid, esters like parabens, and quaternary ammonium compounds.