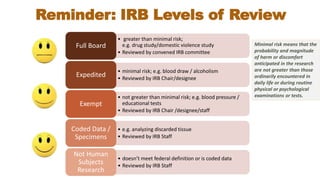
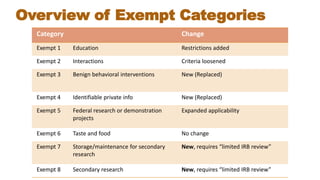
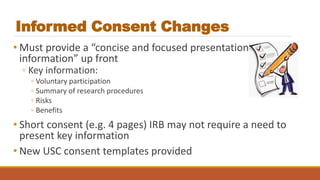
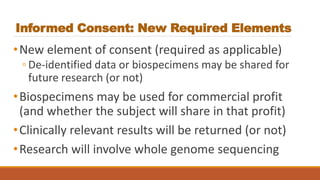
This document summarizes key changes to the Common Rule regulations for social and behavioral research. It discusses revisions to definitions of terms like intervention and clinical trial. It outlines changes to continuing review requirements and informed consent elements. Exemption categories were heavily revised, with new exemptions for benign behavioral interventions and secondary use of identifiable data/biospecimens requiring limited IRB review. Single IRB review is now required for multisite studies funded by NIH or HHS. Resources for learning about the final rule and its implementation are provided.