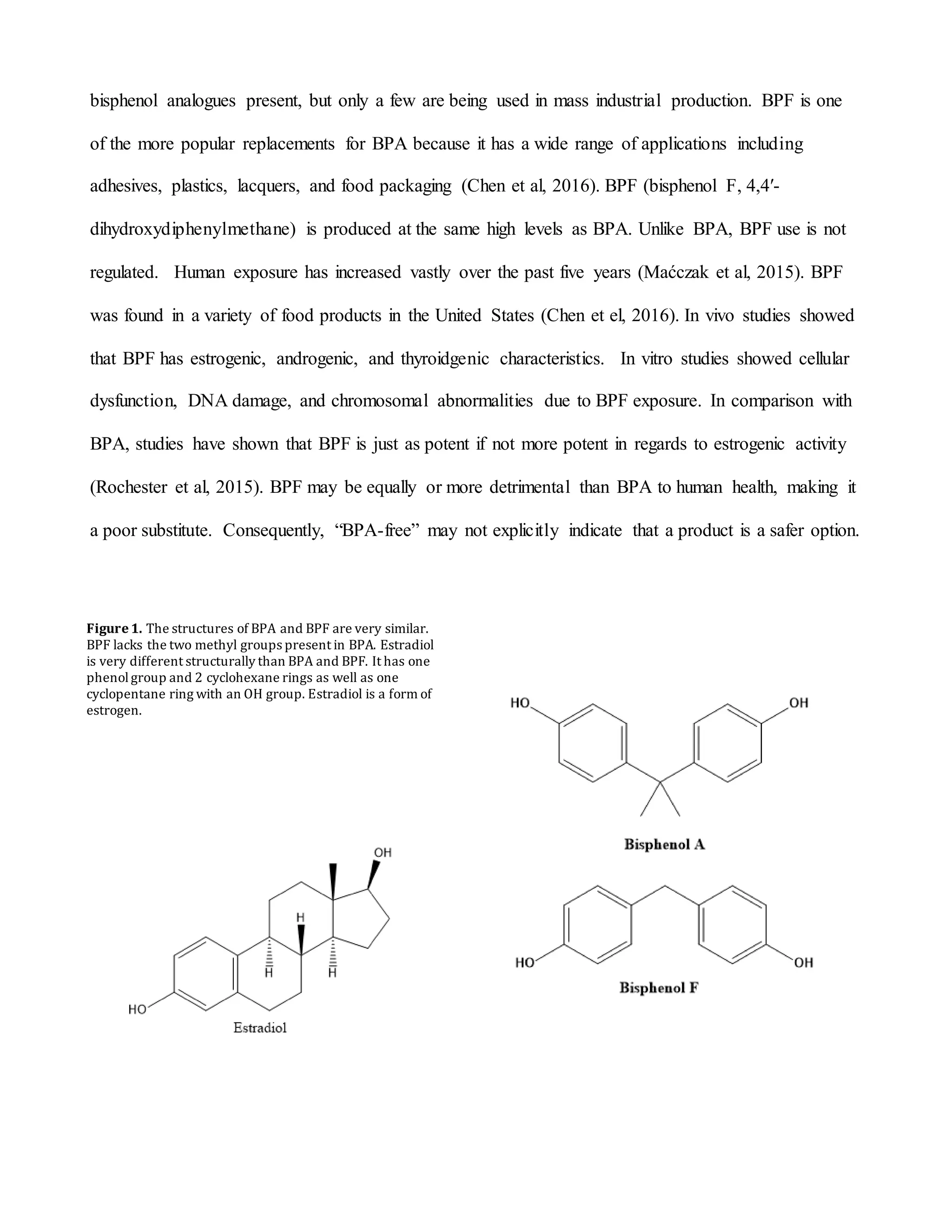
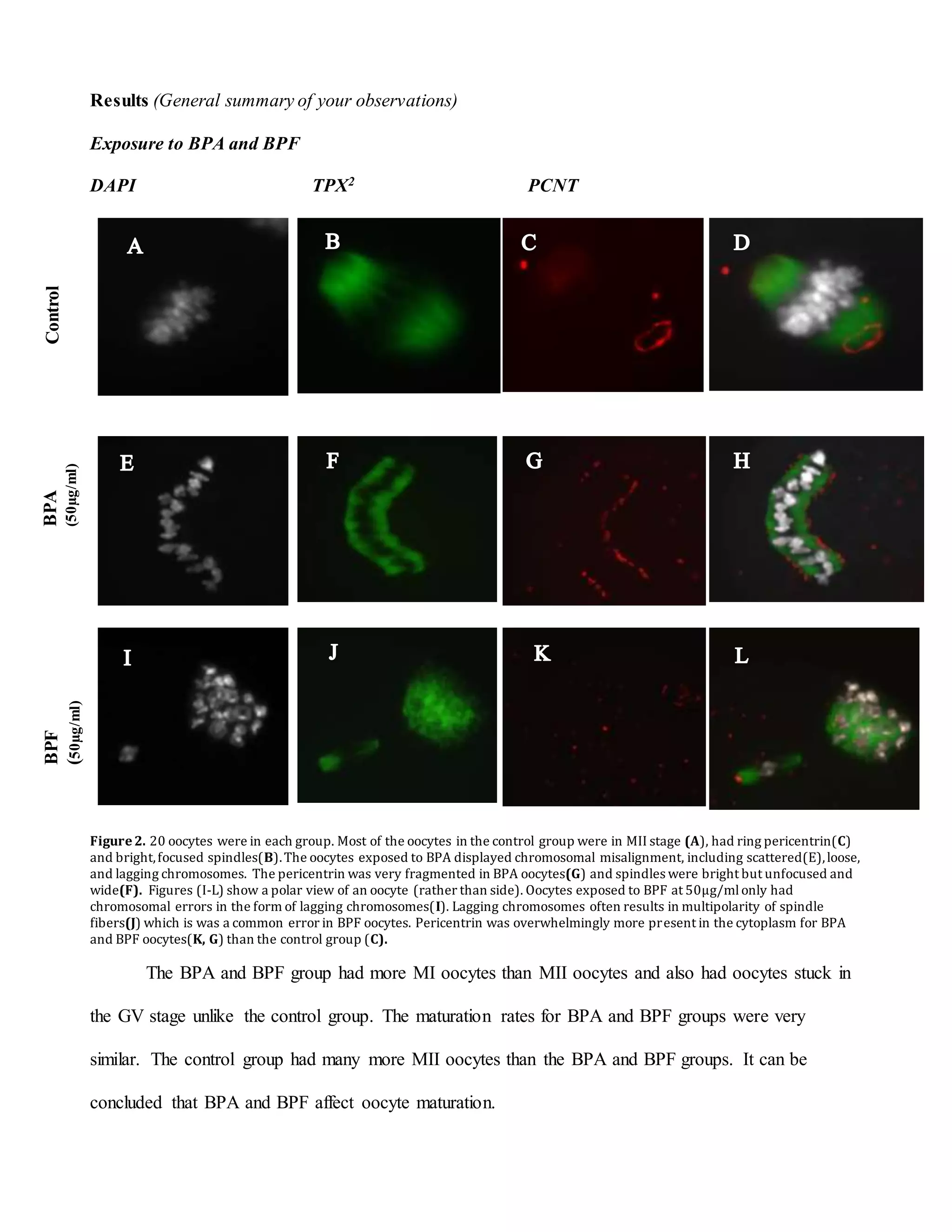
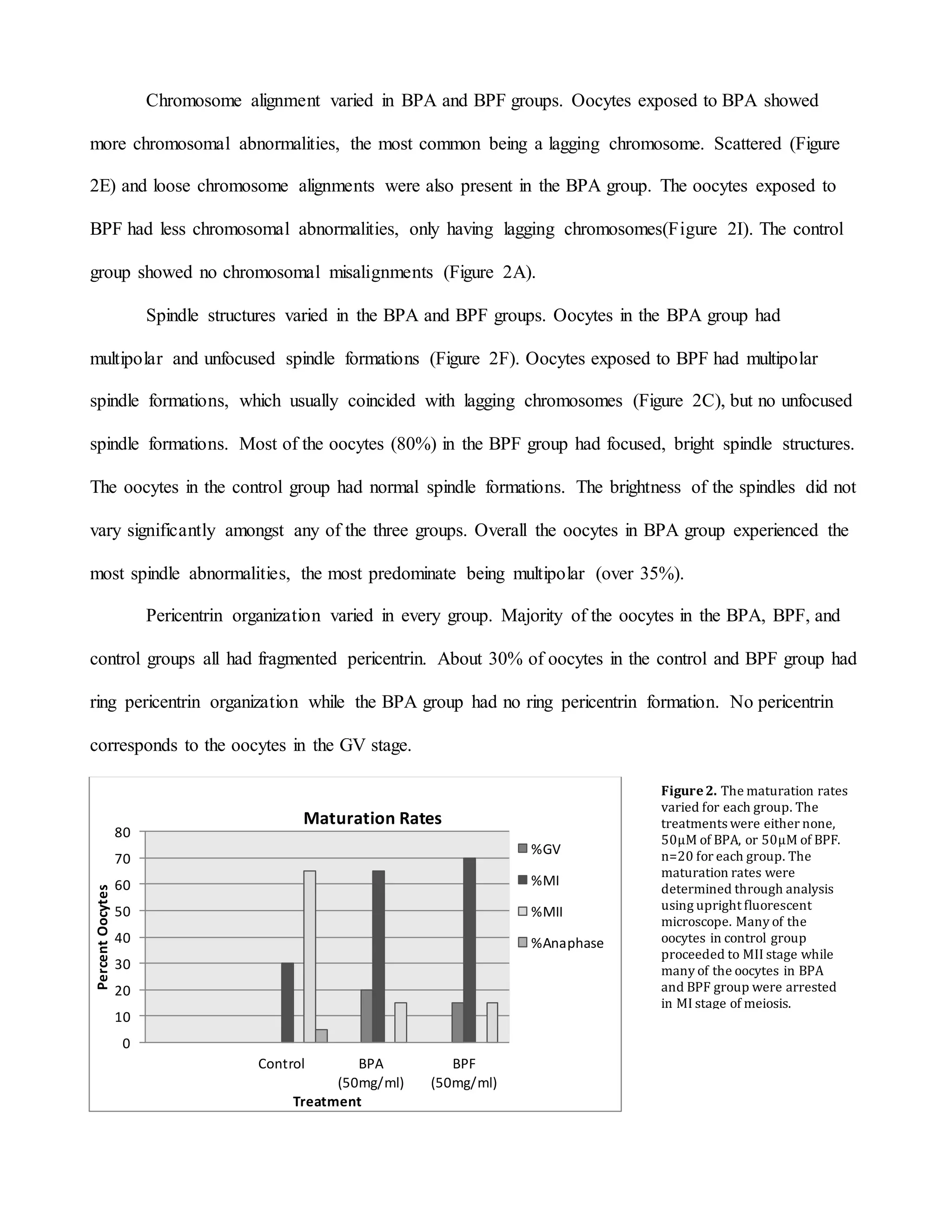
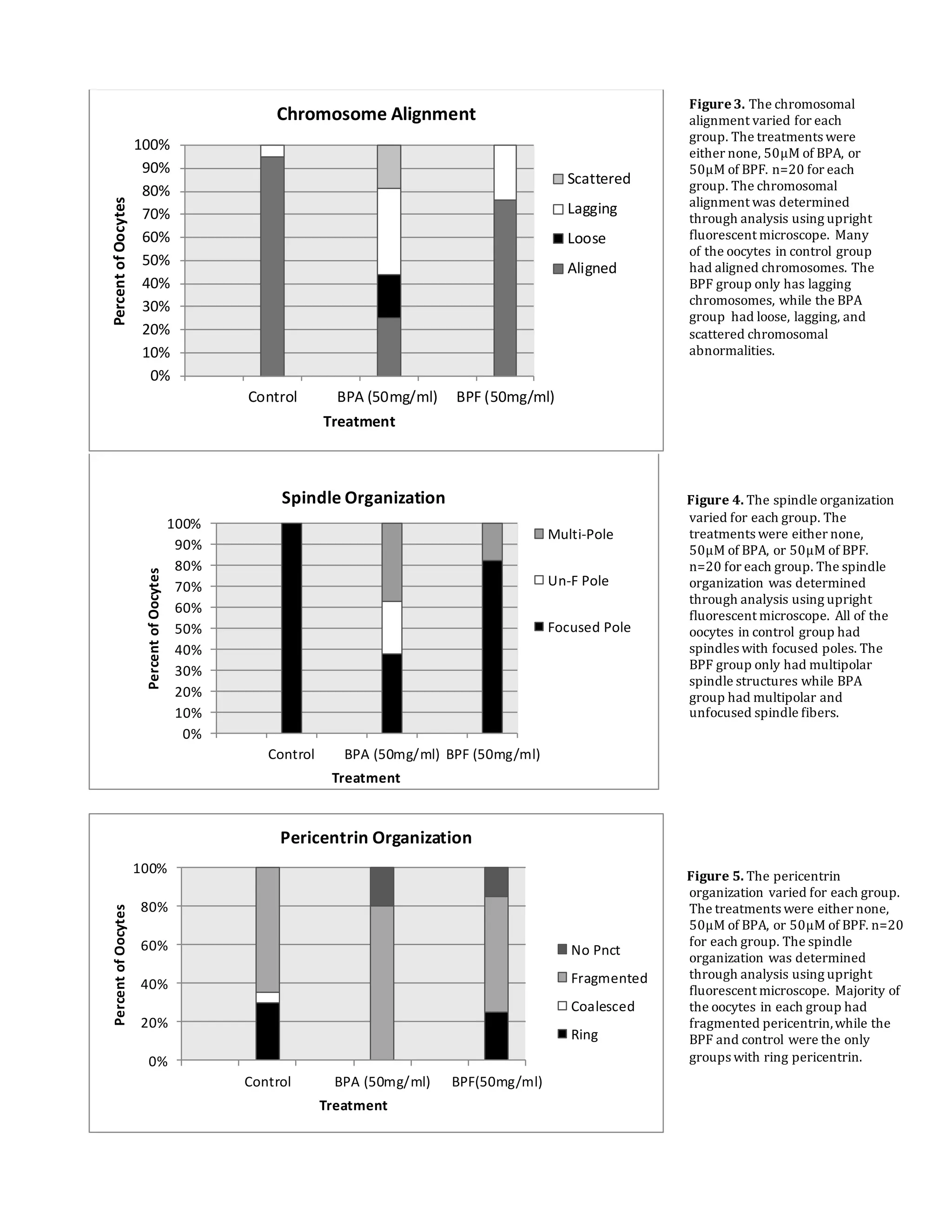
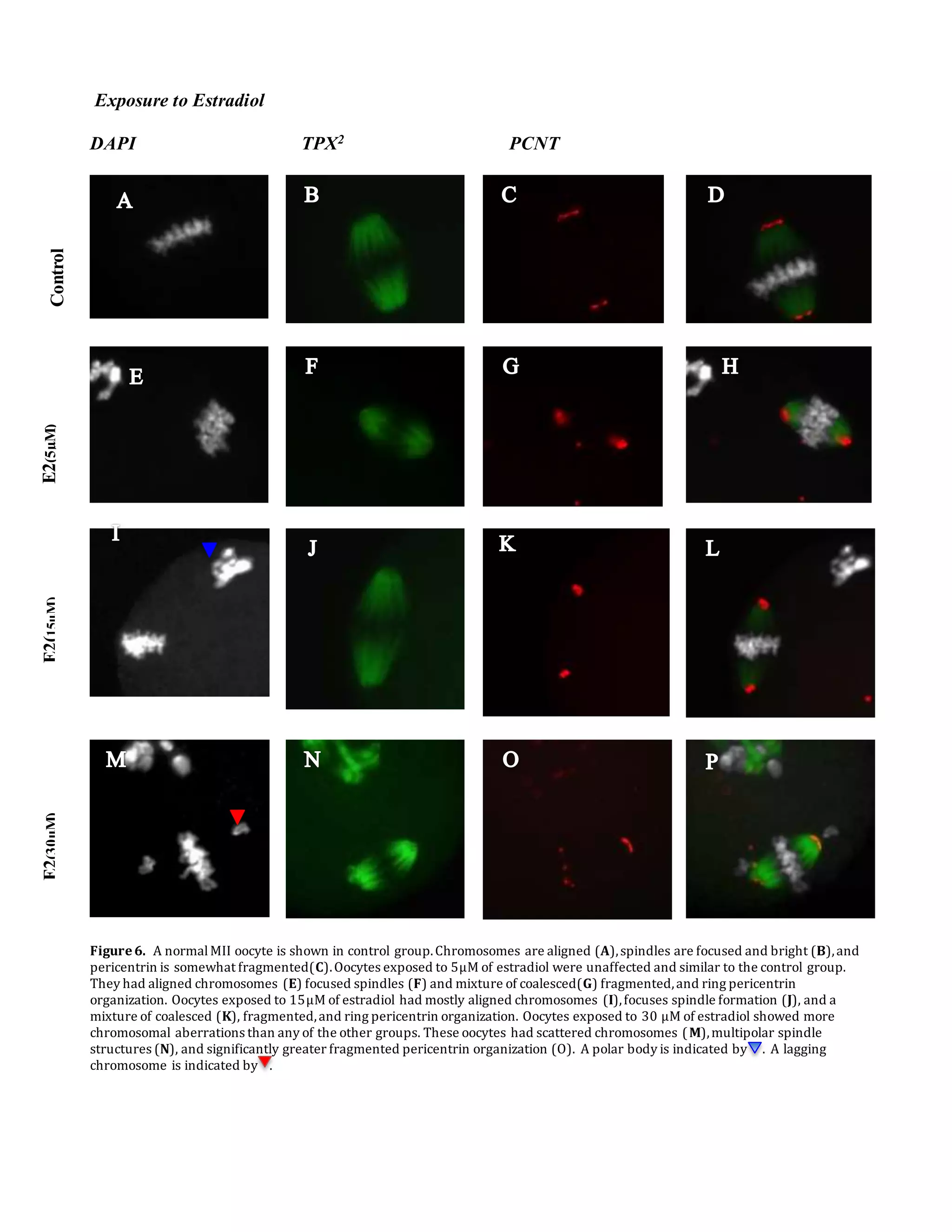
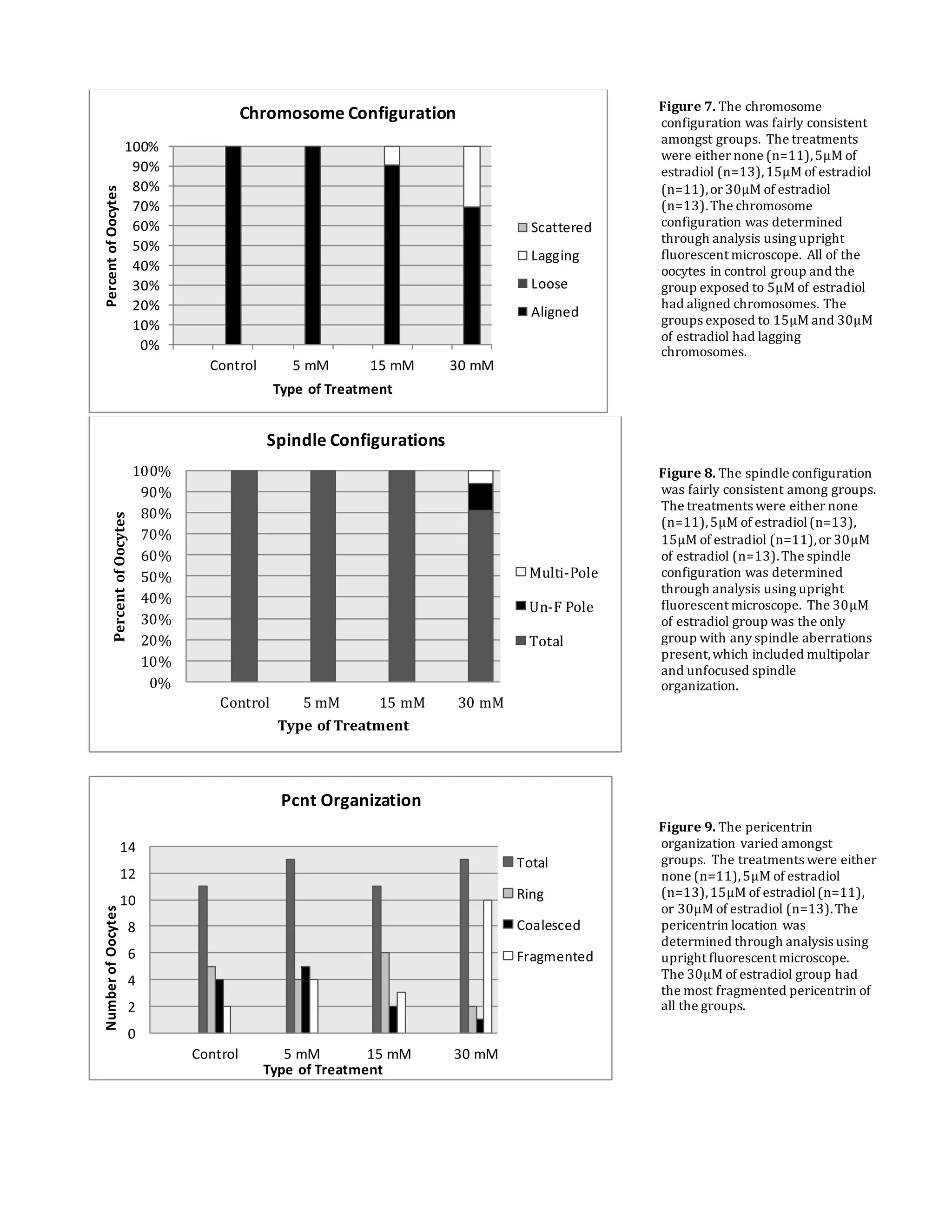
This document summarizes a study that examined the effects of bisphenol A (BPA), bisphenol F (BPF), and high levels of estrogen on oocyte maturation and quality. Mouse oocytes were exposed to varying concentrations of BPA, BPF, and estrogen. The oocytes were then analyzed for spindle formation, centrosome position, and chromosome alignment. Oocytes exposed to BPA, BPF, and high estrogen showed lower maturation rates and more chromosome abnormalities compared to the control group. BPA exposure resulted in more chromosomal errors than BPF exposure. The findings suggest that exposure to synthetic estrogens like BPA and BPF can negatively impact oocyte development and potentially cause birth defects.