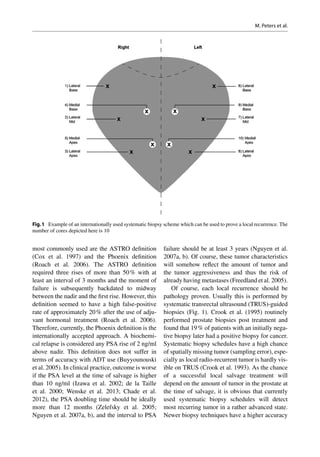
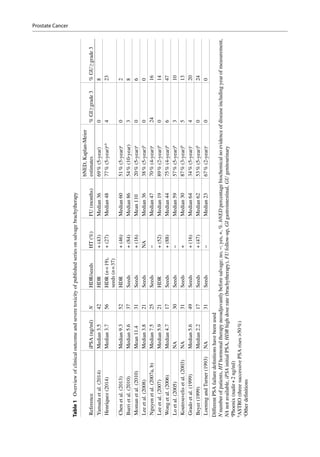
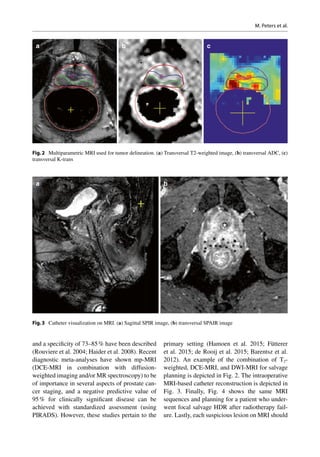
This document discusses salvage radiotherapy for locally recurrent prostate cancer after primary radiation. It notes that while salvage brachytherapy is commonly used, studies have shown high rates of severe toxicity and disappointing cancer control. New diagnostic techniques like MRI and PET scans, along with improved biopsy methods, now allow localization of recurrent tumors, enabling focal salvage techniques. This is expected to reduce toxicity while maintaining cancer control. The document provides selection criteria for salvage treatment, noting it may benefit carefully selected patients with pathology-proven local recurrence at least 2-3 years after primary treatment and limited tumor presentation.











![Das P, Chen MH, Valentine K et al (2002) Using the mag-
nitude of PSA bounce after MRI-guided prostate
brachytherapy to distinguish recurrence, benign pre-
cipitating factors, and idiopathic bounce. Int J Radiat
Oncol Biol Phys 54:698–702
de Castro Abreu AL, Bahn D, Leslie S et al (2013)
Salvage focal and salvage total cryoablation for locally
recurrent prostate cancer after primary radiation ther-
apy. BJU Int 112:298–307
De la Taille A, Hayek O, Benson MC et al (2000) Salvage
cryotherapy for recurrent prostate cancer after radiation
therapy: the Columbia experience. Urology
55:79–84
de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers
MM (2015) Accuracy of magnetic resonance imaging
for local staging of prostate cancer: a diagnostic meta-
analysis. Eur Urol [epub ahead of print]
Dutch Cancer Society (2010) SCK rapport kanker in
Nederland, pp 141–145. www.kwfkankerbestrijding.
nl/index.jsp?objectid=kwfredactie:6419
Eggener SE, Scardino PT et al (2007) Focal therapy for
localized prostate cancer: a critical appraisal of ratio-
nale and modalities. J Urol 178:2260–2267
Eisenberg ML, Shinohara K (2008) Partial salvage cryo-
ablation of the prostate for recurrent prostate cancer
after radiotherapy failure. Urology 72:1315–1318
Evangelista L, Zattoni F, Guttilla A et al (2013) Choline
PET or PET/CT and biochemical relapse of prostate
cancer: a systematic review and meta-analysis. Clin
Nucl Med 38:305–314
Freedland SJ, Humphreys EB, Mangold LA et al (2005)
Risk of prostate cancer-specific mortality following
biochemical recurrence after radical prostatectomy.
JAMA 294:433–439
Fütterer JJ, Briganti A, De Visschere P et al (2015) Can
clinically significant prostate cancer be detected with
multiparametric magnetic resonance imaging? A sys-
tematic review of the literature. Eur Urol 68:
1045–1053
Gelet A, Chapelon JY, Poissonnier L et al (2004) Local
recurrence of prostate cancer after external beam
radiotherapy: early experience of salvage therapy
using high-intensity focused ultrasonography. Urology
63:625–629
Grado GL, Collins JM, Kriegshauser JS et al (1999)
Salvage brachytherapy for localized prostate cancer
after radiotherapy failure. Urology 53:2–10
Grignon DJ, Hammond EH (1995) College of American
Pathologists Conference XXVI on clinical relevance
of prognostic markers in solid tumors. Report of the
prostate cancer working group. Arch Pathol Lab Med
119:1122–1126
Haider MA, Chung P, Sweet J et al (2008) Dynamic
contrast-enhanced magnetic resonance imaging for
localization of recurrent prostate cancer after external
beam radiotherapy. Int J Radiat Oncol Biol Phys
70:425–430
Hamoen EH, de Rooij M, Witjes JA, Barentsz JO, Rovers
MM (2015) Use of the prostate imaging reporting and
data system (PI-RADS) for prostate cancer detection
with multiparametric magnetic resonance imaging: a
diagnostic meta-analysis. Eur Urol 67:1112–1121
Heesakkers RA, Hövels AM, Jager GJ, van den Bosch
HC, Witjes JA, Raat HP, Severens JL, Adang EM, van
der Kaa CH, Fütterer JJ, Barentsz J (2008) MRI with a
lymph-node-specific contrast agent as an alternative to
CT scan and lymph-node dissection in patients with
prostate cancer: a prospective multicohort study.
Lancet Oncol 9:850–856
Heidenreich A, Aus G, Bolla M et al (2008) EAU guide-
lines on prostate cancer. Eur Urol 53:68–80
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU
guidelines on prostate cancer. Part II: treatment of
advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol 65:467–479
Hinnen KA, Monninkhof EM, Battermann JJ et al (2012)
Prostate specific antigen bounce is related to overall
survival in prostate brachytherapy. Int J Radiat Oncol
Biol Phys 82:883–888
Henríquez I, Sancho G, Hervás A et al (2014) Salvage
brachytherapy in prostate local recurrence after radia-
tion therapy: predicting factors for control and toxic-
ity. Radiat Oncol 9:102–109
Hovels AM, Heesakkers RA, Adang EM et al (2008) The
diagnostic accuracy of CT and MRI in the staging of
pelvic lymph nodes in patients with prostate cancer: a
meta-analysis. Clin Radiol 63:387–395
Hsu CC, Hsu H, Pickett B et al (2013) Feasibility of MR
imaging/MR spectroscopy-planned focal partial sal-
vage permanent prostate implant (PPI) for localized
recurrence after initial PPI for prostate cancer. Int
J Radiat Oncol Biol Phys 85:370–377
Huang WC, Kuroiwa K, Serio AM et al (2007) The ana-
tomical and pathological characteristics of irradiated
prostate cancers may influence the oncological efficacy
of salvage ablative therapies. J Urol 177:1324–1329
Huang SP, Bao BY, Wu MT et al (2011) Impact of prostate-
specific antigen (PSA) nadir and time to PSA nadir on
disease progression in prostate cancer treated with
androgen-deprivation therapy. Prostate 71:1189–1197
Izawa JI, Madsen LT, Scott SM et al (2002) Salvage cryo-
therapy for recurrent prostate cancer after radiother-
apy: variables affecting patient outcome. J Clin Oncol
20:2664–2671
Jadvar H (2015) PSMA PET in prostate cancer. J Nucl
Med 56:1131–1132
Kalapurakal JA, Mittal BB, Sathiaseelan V (2001)
Re-irradiation and external hyperthermia in locally
advanced, radiation recurrent, hormone refractory pros-
tate cancer: a preliminary report. Br J Radiol 74:745–751
Kanthabalan A, Shah T, Arya M et al (2015) The
FORECAST study – focal recurrent assessment and
salvage treatment for radiorecurrent prostate cancer.
Contemp Clin Trials [epub ahead of print]
Kimura M, Mouraviev V, Tsivian M, Mayes JM, Satoh T,
Polascik TJ (2010) Current salvage methods for recur-
rent prostate cancer after failure of primary radiother-
apy. BJU Int 150:191–201
Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw
A (2010) Clinical results of long-term follow-up of a
Prostate Cancer](https://image.slidesharecdn.com/db0cea79-5eee-4b1e-95b8-d54a57bc05eb-160510172419/85/Re-irradiation-Prostate-Cancer-15-320.jpg)

![Pisters LL, English SF, Scott SM et al (2000) Salvage
prostatectomy with continent catheterizable urinary
reconstruction: a novel approach to recurrent prostate
cancer after radiation therapy. J Urol 163:1771–1774
Pound CR, Brawer MK, Partin AW (2001) Evaluation and
treatment of men with biochemical prostate-specific
antigen recurrence following definitive therapy for
clinically localized prostate cancer. Rev Urol 3:72–84
Prestigiacomo AF, Stamey TA (1996) Physiological varia-
tion of serum prostate specific antigen in the 4.0 to
10.0 Ng/ml range in male volunteers. J Urol
155:1977–1980
Pucar D, Sella T, Schoder H (2008) The role of imaging in
the detection of prostate cancer local recurrence after
radiation therapy and surgery. Curr Opin Urol 18:87–97
Roach M III, Hanks G, Thames H Jr, Schellhammer P,
Shipley WU, Sokol GH et al (2006) Defining bio-
chemical failure following radiotherapy with or with-
out hormonal therapy in men with clinically localized
prostate cancer: recommendations of the RTOG-
ASTRO Phoenix consensus conference. Int J Radiat
Oncol Biol Phys 65:965–974
Rouviere O, Valette O, Grivolat S et al (2004) Recurrent
prostate cancer after external beam radiotherapy:
value of contrast-enhanced dynamic MRI in localizing
intraprostatic tumor–correlation with biopsy findings.
Urology 63:922–927
Rybalov M, Ananias HJK, Hoving HD (2014) PSMA,
EpCAM, VEGF and GRPR as imaging targets
in locally recurrent prostate cancer after radiotherapy.
Int J Mol Sci 15:6046–6061
Sanderson KM, Penson DF, Cai J et al (2006) Salvage
radical prostatectomy: quality of life outcomes and
long-term oncological control of radiorecurrent pros-
tate cancer. J Urol 176:2025–2031
Sasaki H, Kido M, Miki K et al (2013) Salvage partial
brachytherapy for prostate cancer recurrence after pri-
mary brachytherapy. Int J Urol 21:572–577
Scardino PT (1983) The prognostic significance of biop-
sies after radiotherapy for prostatic cancer. Semin Urol
1:243–252
Sheets NC, Goldin GH, Meyer AM et al (2012) Intensity-
modulated radiation therapy, proton therapy, or confor-
mal radiation therapy and morbidity and disease control
in localized prostate cancer. JAMA 307:1611–1620
Siddiqui MM, Rais-Bahrami S, Turkbey B et al (2015)
Comparison of MR/ultrasound fusion-guided biopsy
with ultrasound-guided biopsy for the diagnosis of
prostate cancer. JAMA 313:390–397
Sivaraman A, Sanchez-Salas R, Barret E (2015)
Transperineal template-guided mapping biopsy of the
prostate. Int J Urol 22:146–151
Spiess PE, Katz AE, Chin JL et al (2010) A pretreatment
nomogram predicting biochemical failure after sal-
vage cryotherapy for locally recurrent prostate cancer.
BJU Int 106:194–198
Stephenson AJ, Scardino PT, Bianco FJ et al (2004)
Morbidity and functional outcomes of salvage radical
prostatectomy for locally recurrent prostate cancer
after radiation therapy. J Urol 172:2239–2243
Tran H, Kwok J, Pickles T, Tyldesley S, Black PC (2014)
Underutilization of local salvage therapy after radiation
therapy for prostate cancer. Urol Oncol 32:701–706
Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann
LM (2013) The role of 11C-choline and
18Ffluorocholine positron emission tomography
(PET) and PET/CT in prostate cancer: a systematic
review and meta-analysis. Eur Urol 64:106–117
Vaidya A, Soloway MS (2000) Salvage radical prostatec-
tomy for radiorecurrent prostate cancer: morbidity
revisited. J Urol 164:1998–2001
Valerio M, Anele C, Charman SC et al (2015) Transperineal
template prostate mapping biopsies: an evaluation of
different protocols in detection of clinically significant
prostate cancer. BJU Int [epub ahead of print]
Van den Bos W, Muller BG, de Bruin DM (2015) Salvage
ablative therapy in prostate cancer: international mul-
tidisciplinary consensus on trial design. Urol Oncol
33:495.e1–495.e7
Van der Heide UA, Dehnad H, Hofman P, Kotte ANTJ,
Lagendijk JJW, Van Vulpen M (2007) Analysis of
fiducial marker based position verification in the
intensity-modulated radiotherapy of patients with
prostate cancer. Radiother Oncol 82:38–45
Van der Poel HG, Beetsma DB, van Boven H et al (2007)
Perineal salvage prostatectomy for radiation resistant
prostate cancer. Eur Urol 51:1565–1571
Wallner KE, Nori D, Morse MJ et al (1990) 125iodine
reimplantation for locally progressive prostatic carci-
noma. J Urol 144:704–706
Wang H, Vees H, Miralbell R, Wissmeyer M, Steiner C,
Ratib O, Senthamizhchelvan S, Zaidi H (2009)
18F-fluorocholine PET-guided target volume delinea-
tion techniques for partial prostate re-irradiation in local
recurrent prostate cancer. Radiother Oncol 93:220–225
Ward JF, Sebo TJ, Blute ML et al (2005) Salvage surgery
for radiorecurrent prostate cancer: contemporary out-
comes. J Urol 173:1156–1160
Ward JF, Pagliaro LC, Pisters LL et al (2008) Salvage
therapy for radiorecurrent prostate cancer. Curr Probl
Cancer 32(6):242–271
Wenske S, Quarrier S, Katz AE (2013) Salvage cryosur-
gery of the prostate for failure after primary radiother-
apy or cryosurgery: long-term clinical, functional, and
oncologic outcomes in a large cohort at a tertiary
referral centre. Eur Urol 64:1–7
Williams AK, Martinez CH, Lu C, Ng CK, Pautler SE,
Chin JL (2011) Disease-free survival following sal-
vage cryotherapy for biopsy-proven radio-recurrent
prostate cancer. Eur Urol 60:405–410
Wong WW, Buskirk SJ, Schild SE et al (2006) Combined
prostate brachytherapy and short-term androgen depri-
vation therapy as salvage therapy for locally recurrent
prostate cancer after external beam irradiation. J Urol
176:2020–2024
Yamada Y, Kollmeier MA, Pei X et al (2014) A phase II
study of salvage high-dose-rate brachytherapy for the
treatment of locally recurrent prostate cancer after
definitive external beam radiotherapy. Brachytherapy
13:111–116
Prostate Cancer](https://image.slidesharecdn.com/db0cea79-5eee-4b1e-95b8-d54a57bc05eb-160510172419/85/Re-irradiation-Prostate-Cancer-17-320.jpg)
