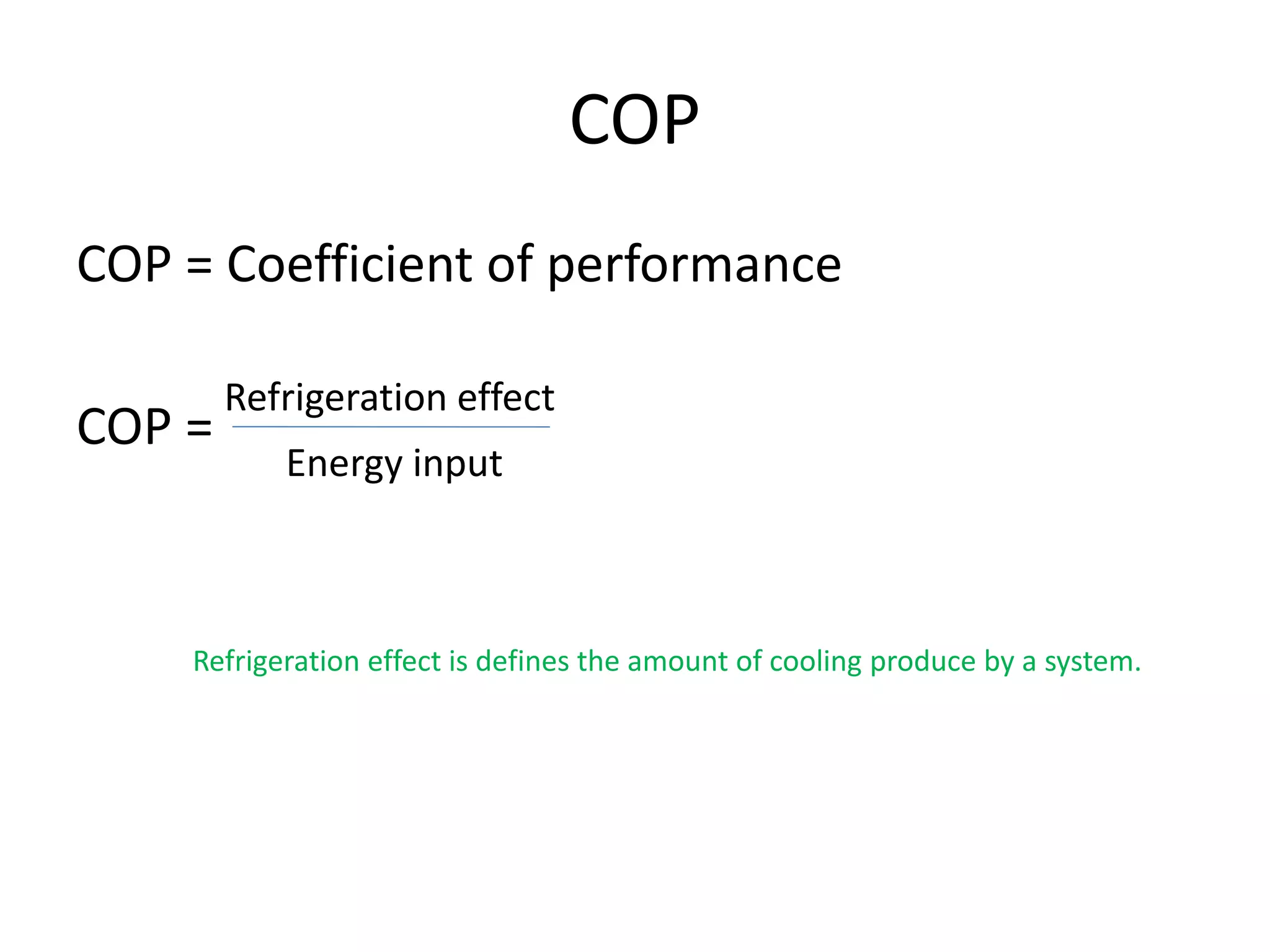
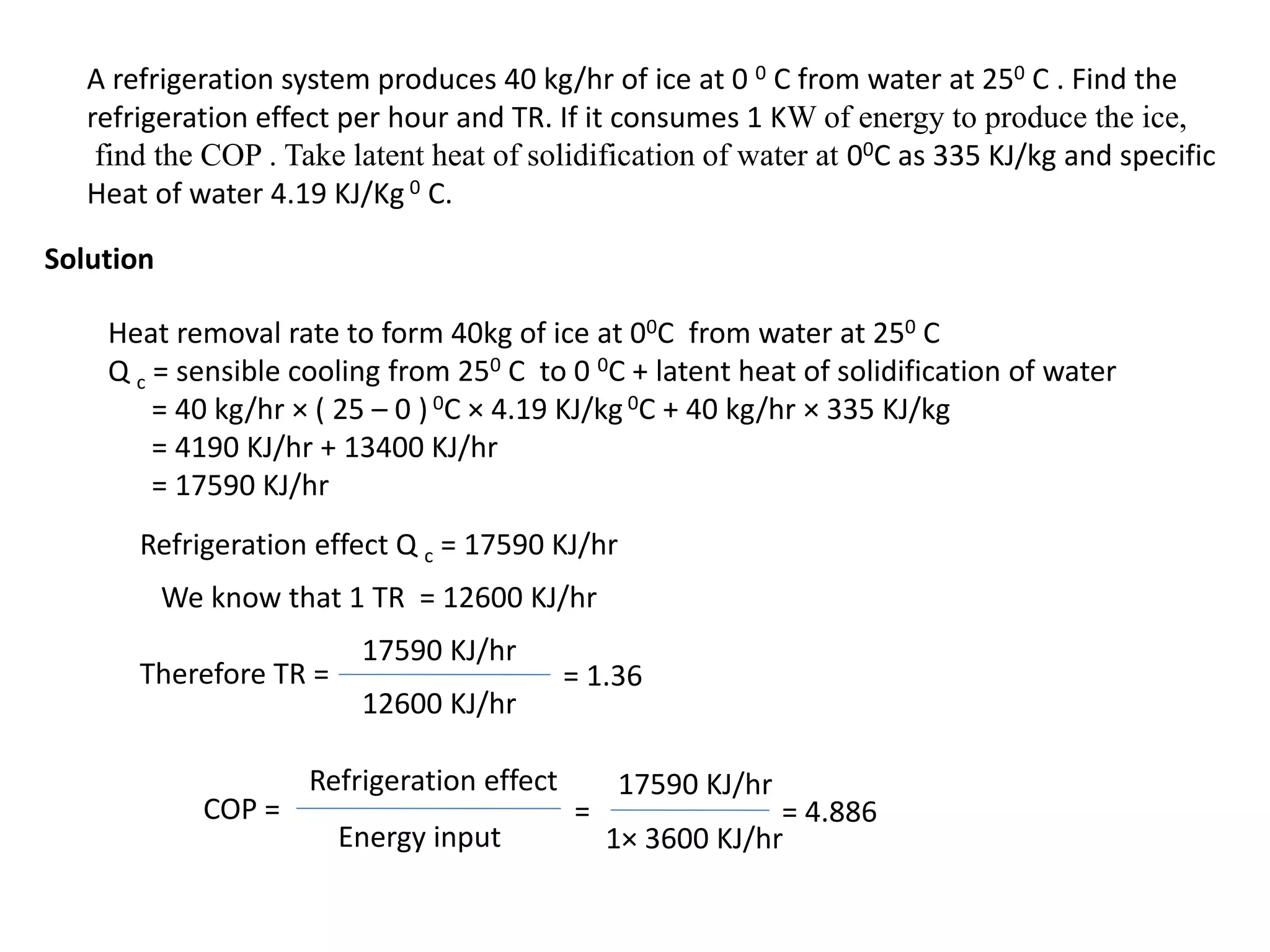
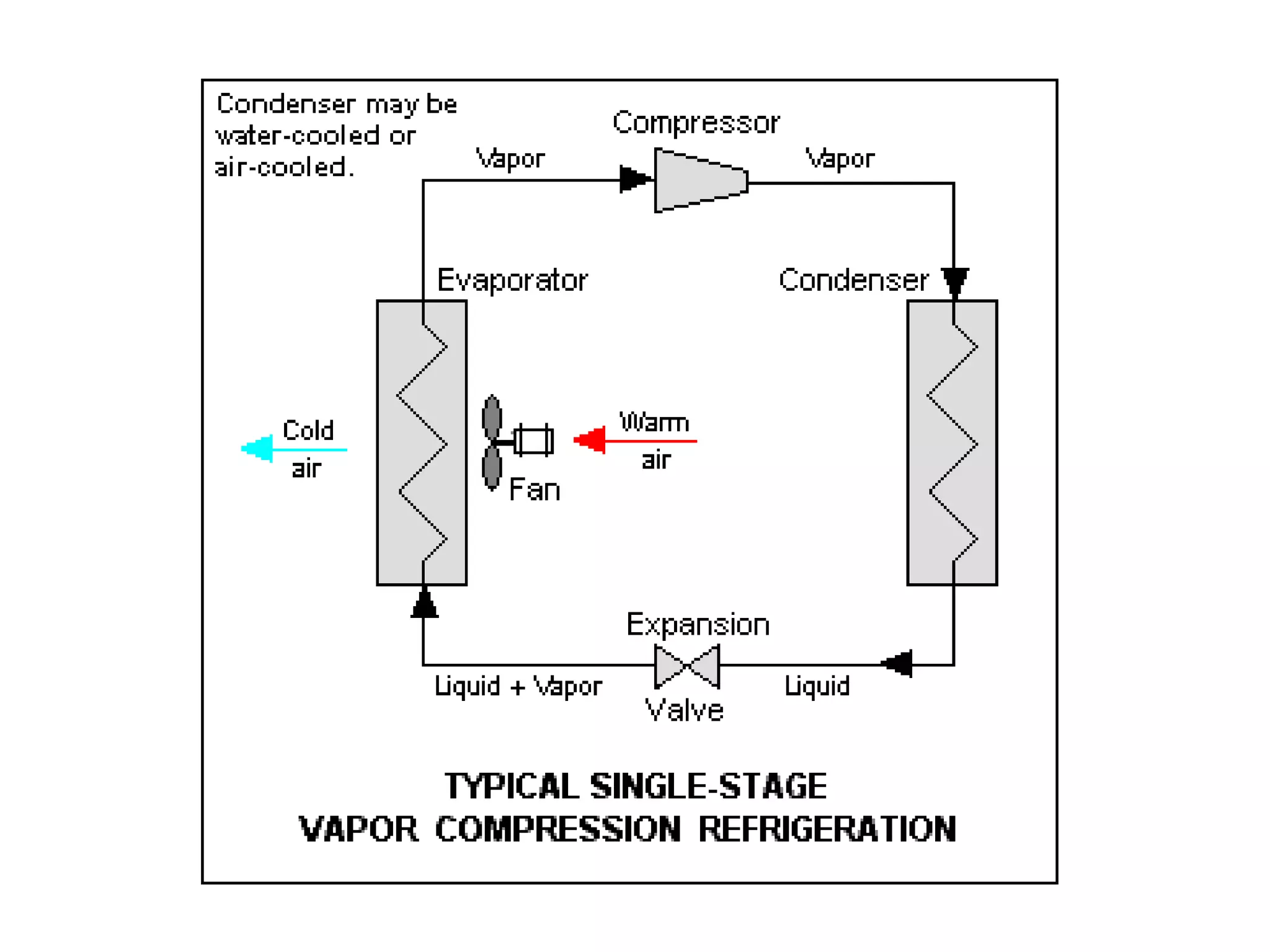
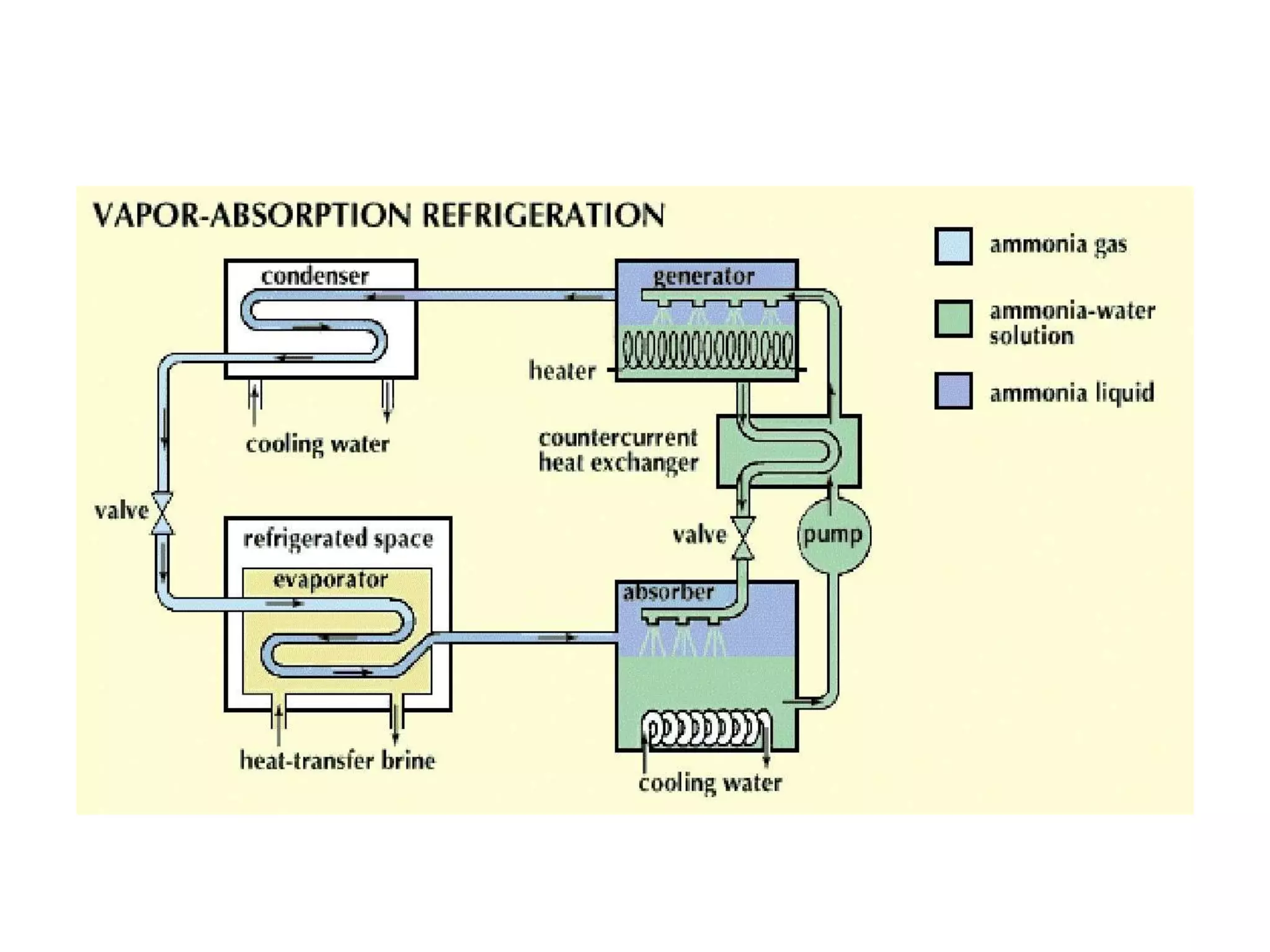
The document outlines refrigeration and air conditioning, defining refrigeration as removing unwanted heat and air conditioning as controlling temperature, humidity, and air quality. It details applications of air conditioning and the standard unit of refrigeration, ton refrigeration (tr), along with calculations for refrigeration effect and coefficient of performance (COP). Additionally, it describes types of refrigeration systems like vapor compression and vapor absorption, along with ideal refrigerant properties and refrigeration cycles.