Reaction Between Hydrogen and Chlorine.pptx
•Download as PPTX, PDF•
0 likes•30 views
The document summarizes reaction mechanisms for the reaction of molecular hydrogen with chlorine and iodine. For the hydrogen-chlorine reaction, a chain reaction mechanism is proposed involving initiation, propagation, and termination steps. For the hydrogen-iodine reaction, it was originally thought to be bimolecular but is shown to involve radical intermediates and proceed by a chain mechanism as well, though multiple mechanisms may contribute to the overall reaction.
Report
Share
Report
Share
Recommended
Recommended
More Related Content
Similar to Reaction Between Hydrogen and Chlorine.pptx
Similar to Reaction Between Hydrogen and Chlorine.pptx (9)
Recently uploaded
Recently uploaded (20)
ESR_factors_affect-clinic significance-Pathysiology.pptx

ESR_factors_affect-clinic significance-Pathysiology.pptx
In silico drugs analogue design: novobiocin analogues.pptx

In silico drugs analogue design: novobiocin analogues.pptx
SAMPLING.pptx for analystical chemistry sample techniques

SAMPLING.pptx for analystical chemistry sample techniques
Astronomy Update- Curiosity’s exploration of Mars _ Local Briefs _ leadertele...

Astronomy Update- Curiosity’s exploration of Mars _ Local Briefs _ leadertele...
Earliest Galaxies in the JADES Origins Field: Luminosity Function and Cosmic ...

Earliest Galaxies in the JADES Origins Field: Luminosity Function and Cosmic ...
BLOOD AND BLOOD COMPONENT- introduction to blood physiology

BLOOD AND BLOOD COMPONENT- introduction to blood physiology
FAIRSpectra - Towards a common data file format for SIMS images

FAIRSpectra - Towards a common data file format for SIMS images
PRESENTATION ABOUT PRINCIPLE OF COSMATIC EVALUATION

PRESENTATION ABOUT PRINCIPLE OF COSMATIC EVALUATION
insect taxonomy importance systematics and classification

insect taxonomy importance systematics and classification
GEOLOGICAL FIELD REPORT On Kaptai Rangamati Road-Cut Section.pdf

GEOLOGICAL FIELD REPORT On Kaptai Rangamati Road-Cut Section.pdf
GLOBAL AND LOCAL SCENARIO OF FOOD AND NUTRITION.pptx

GLOBAL AND LOCAL SCENARIO OF FOOD AND NUTRITION.pptx
National Biodiversity protection initiatives and Convention on Biological Di...

National Biodiversity protection initiatives and Convention on Biological Di...
Gliese 12 b, a temperate Earth-sized planet at 12 parsecs discovered with TES...

Gliese 12 b, a temperate Earth-sized planet at 12 parsecs discovered with TES...
The importance of continents, oceans and plate tectonics for the evolution of...

The importance of continents, oceans and plate tectonics for the evolution of...
Reaction Between Hydrogen and Chlorine.pptx
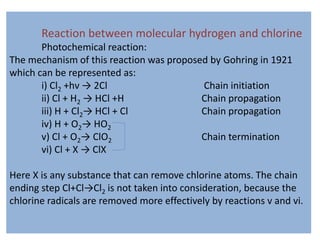
- 1. Reaction between molecular hydrogen and chlorine Photochemical reaction: The mechanism of this reaction was proposed by Gohring in 1921 which can be represented as: i) Cl2 +hν → 2Cl Chain initiation ii) Cl + H2 → HCl +H Chain propagation iii) H + Cl2→ HCl + Cl Chain propagation iv) H + O2→ HO2 v) Cl + O2→ ClO2 Chain termination vi) Cl + X → ClX Here X is any substance that can remove chlorine atoms. The chain ending step Cl+Cl→Cl2 is not taken into consideration, because the chlorine radicals are removed more effectively by reactions v and vi.
- 2. The concentration of chlorine atom[Cl] in this mechanism is much lower than that of [Br], since the activation energy of Cl is lower than Br. The rate of Cl formation in step (i) is 2I, where I is the intensity of the absorbed light , because two radicals are formed for each absorbed photon. Applying the steady-state treatment to chlorine atoms, we get: d[Cl] /dt = 2I-k2[Cl][H2] +k3[H][Cl2] – k5[Cl][O2] – k6[Cl][X]=0….1 The steady-state equation for H atoms is: d[H]/dt =k2 [Cl][H2]- k3[H][Cl2] – k4[H][O2] =0 …………….2 from which it follows that [Cl]= k3[H][Cl2] + k4[H][O2] / k2[H2] ……………..3 Introduction the above expression into eq. 1, with some rearrangement and neglecting the small terms involving [O2]2 , gives: [H] = 2Ik2 [H2]/ k3 k6 [X][Cl2] + [O2] k2k4 [H2] + k3k5[Cl2] + k4k6 [X]………4
- 3. The rate of the formation of hydrogen chloride is given by: νHCl =d[HCl]/dt = k2[Cl][H2] + k3[H][Cl2] ………………….5 Substraction eq. 2 gives: νHCl =2 k3[H][Cl2] + k4[H][O2] …………………..6 At low concentration of oxygen the second term can be neglected, and then, with the introduction of eq. 4 we get: νHCl = 2k2k3 [H2][Cl2]I/k3k6[Cl2][X] +[O2] k2k4 [H2]+k3k5[Cl2] + k4k6[X])……7 or νHCl = 2k3 /k4 I [H2][Cl2]/k3k6 / k2k4[Cl2][X] +[O2] [H2]+k3k5/k2k4 [Cl2] + k6/k2[X])……8 Apart from the final term in the denominator, this expression is of the form of the empirical equation i.e. d[HCl]/dt= kI[H2][Cl2]/m [Cl2]+ [Cl2]([H2] +n[Cl2]), the constants being related by: k= 2k3 /k4 ,m= k3k6 / k2k4[X] and n=k3k5/k2k4 .................9 The scheme of reactions therefore gives a kinetic law that is essentially in agreement with the experimental rate equation. For the thermal reaction between H and Cl the rate may be express as: νHCl = d[HCl]/dt=kI[H2][Cl2]2/mI[Cl2] +[O2]([H2]+nI[Cl2]) …………10
- 4. Reaction between molecular hydrogen and iodine The reaction between H2 and I2 was extensively studied by Bodenstein at the end of the nineteenth century. For many years it was considered to be a true bimolecular reaction. However, in 1959, Sullivan showed the presence of free radicals in the system above 600K, leading to the proposed mechanism: I2→2I I+H2 → HI+H 1 H+I2→HI+I i.e. a chain reaction. Once the intermediate HI is formed, it can be deactivated through the reverse process of step (ii). In addition, it is also plausible that there is the formation of a linear transition state {I…..H…..H….I}‡, which relates I and H2I with the products, HI. The following mechanism includes the formation of intermediates of this type
- 5. i) I2↔2I (fast) ii) I+H2 ↔ H2I (fast) iii)H +H2I ↔2HI (slow) where the rate determining step is step (iii) and thus: ν = k3[I][H2I] ……………..2 Inserting the expressions for [I] and [H2I], obtain from the equilibrium processes(i) and (ii), we get: ν = k[I2][H2I] ……………….3 Here, k=k3k2/k1. This mechanism, together with mechanism (1), all appear to make contributions to the overall reaction. Another process that is almost certainly involved proceeds via the elementary step I2+H2→2HI, which proceeds via a transition state with a trapezoidal configuration. This illustrates that a single reaction can often occur by more than one mechanism proceeding simultaneously.