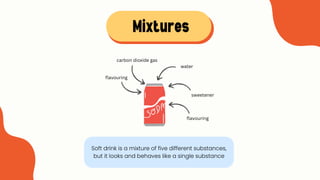
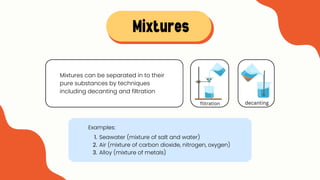
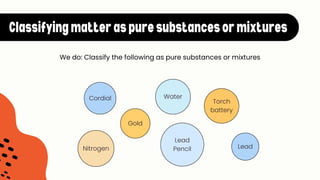
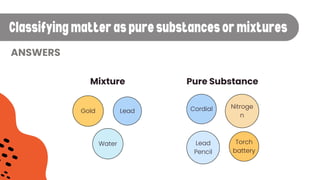
The document explains the concepts of matter, pure substances, and mixtures in chemistry. It defines matter as material that occupies space and has mass, and differentiates pure substances (made up of one type of matter) from mixtures (combinations of two or more pure substances). Examples and classification techniques for mixtures are provided, including heterogeneous and homogeneous mixtures.