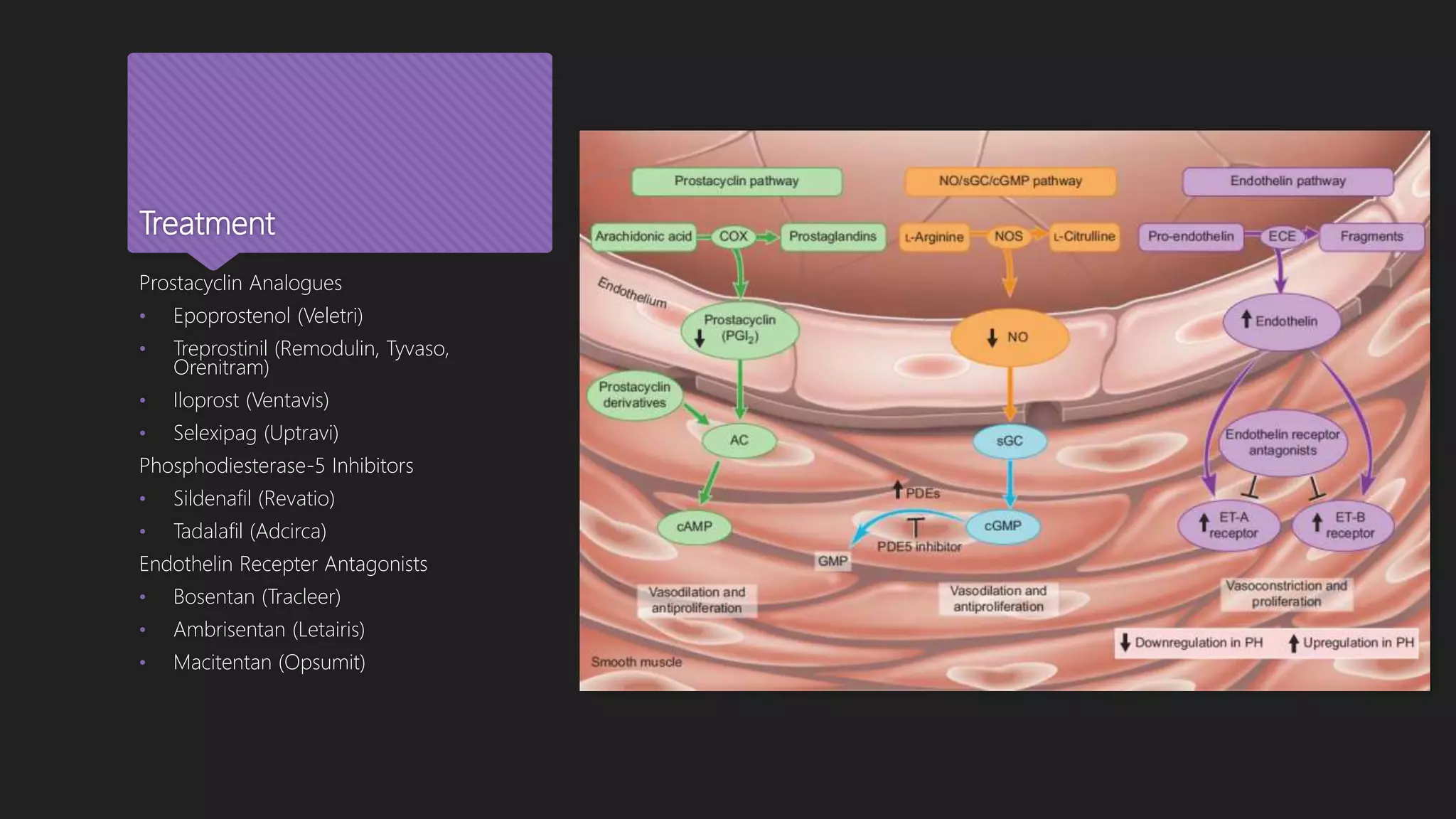
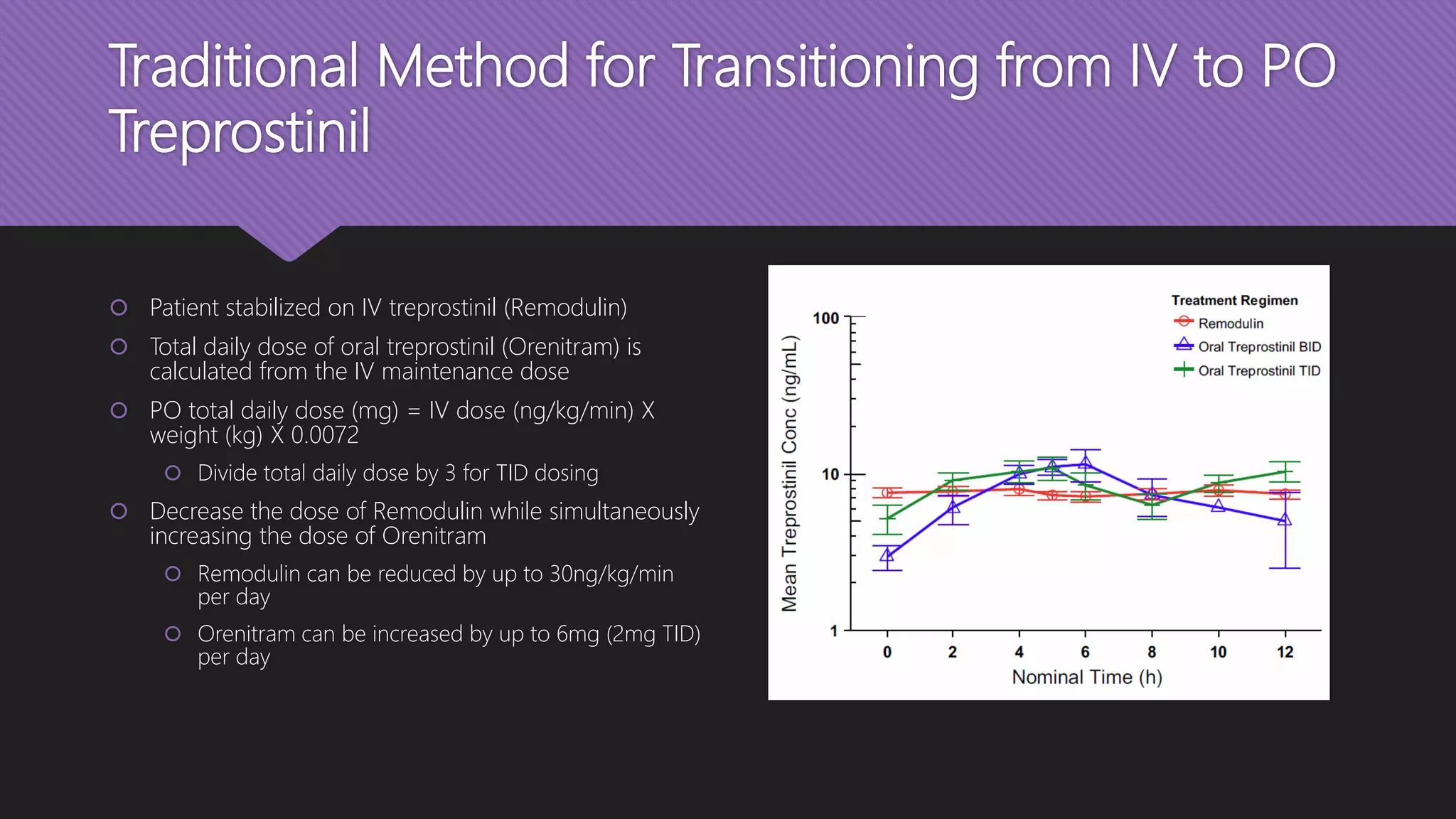
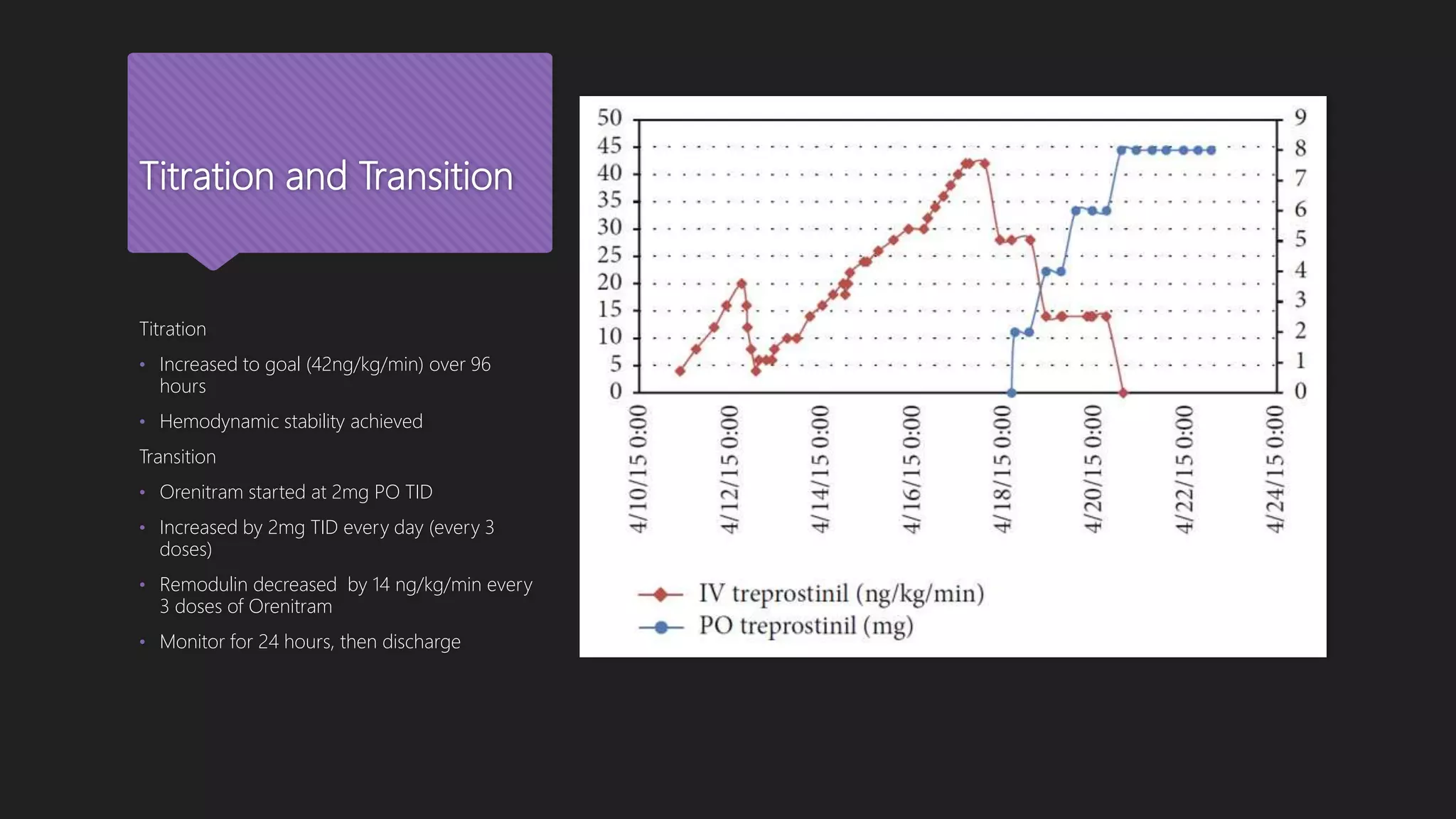
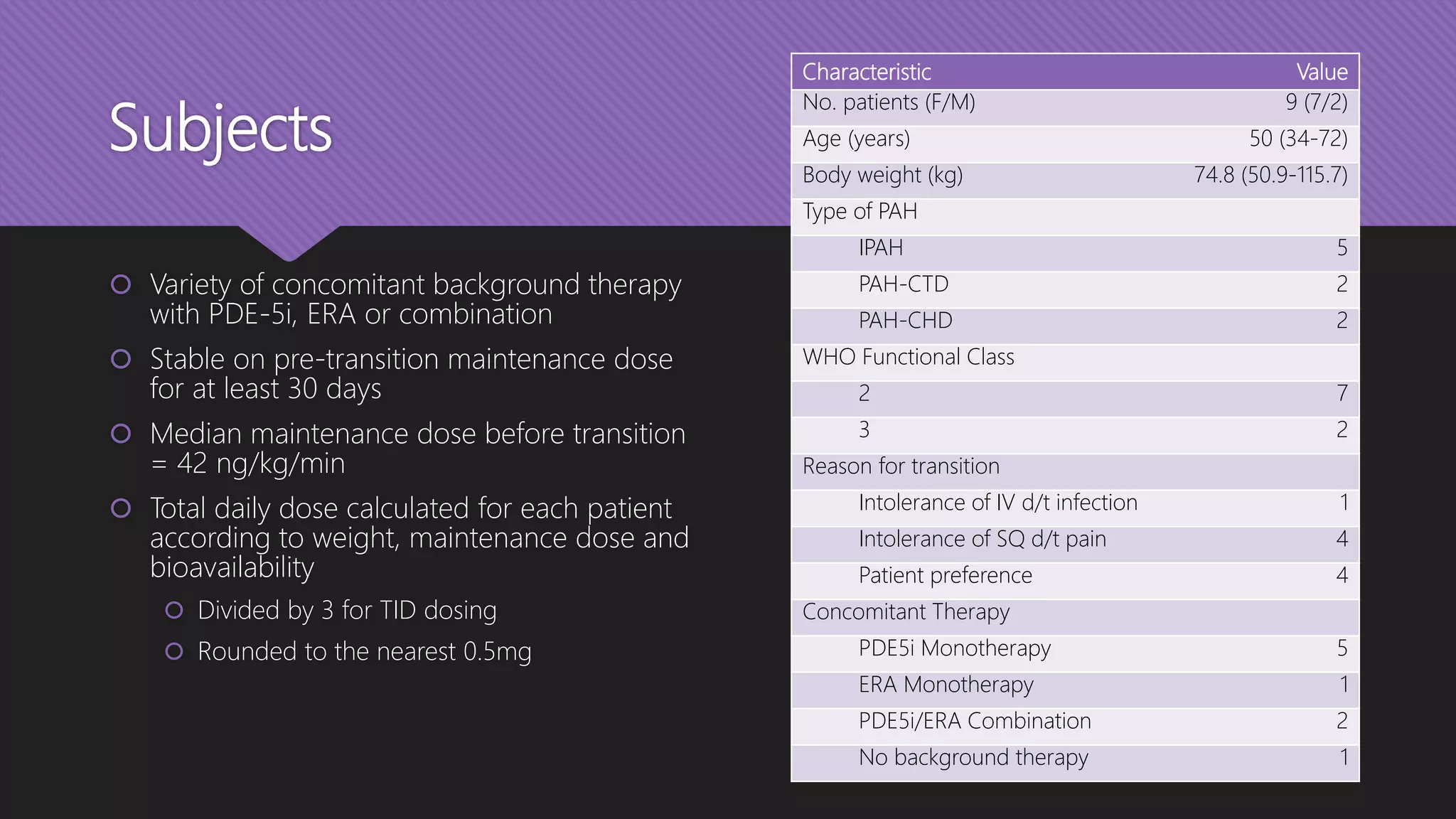
This document provides an outline and overview of transitioning patients from intravenous or inhaled treprostinil (Remodulin) therapy to oral treprostinil (Orenitram) therapy for pulmonary arterial hypertension. It describes a case series that rapidly transitioned 9 stable PAH patients from their current treprostinil treatment to oral Orenitram over 4 days. The results found that the transition was effective for most patients, though 2 required returning to their original therapy due to worsening symptoms.








![References
Coons JC, Miller T, Simon MA, Ishizawar DC, Mathier MA. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients transitioned from parenteral or
inhaled prostacyclins: case series and treatment protocol. Pulmonary Circulation. 2016;6(1):132–5.
Gleason JB, Dolan J, Piran P, Rahaghi FF. The Rapid Initiation, Titration, and Transition from Intravenous to Oral Treprostinil in a Patient with Severe Pulmonary Arterial
Hypertension. Case Reports in Pulmonology. 2015;2015:1–3.
Kumar P, Thudium E, Laliberte K, Zaccardelli D, Nelsen A. A Comprehensive Review of Treprostinil Pharmacokinetics via Four Routes of Administration. Clin Pharmacokinet
Clinical Pharmacokinetics. 2016;
Orenitram (treprostinil) [package insert]. Research Triangle Park, NC: United Therapeutics, 2014.
Pulmonary Arterial Hypertension (PAH) [Internet]. PAH-info.com. Actelion Pharmaceuticals Ltd; 2012 [cited 2016Jun16]. Available from: http://www.pah-info.com/home
Pulmonary Hypertension Fact Sheet [Internet]. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2014 [cited 2016Jun16]. Available from:
http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_pulmonary_hypertension.htm
Taichman DB, Ornelas J, Chung L, Klinger JR, Lewis S, Mandel J, et al. Pharmacologic Therapy for Pulmonary Arterial Hypertension in Adults. Chest. 2014;146(2):449–75.
Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, et al. Causes and Circumstances of Death in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med
American Journal of Respiratory and Critical Care Medicine. 2013;188(3):365–9.
“2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the
European Society of Cardiology (ESC) and the European Respiratory Society (ERS).” Nazzareno Galiè, Marc Humbert, Jean-Luc Vachiery, Simon Gibbs, Irene Lang, Adam
Torbicki, Gérald Simonneau, Andrew Peacock, Anton Vonk Noordegraaf, Maurice Beghetti, Ardeschir Ghofrani, Miguel Angel Gomez Sanchez, Georg Hansmann, Walter
Klepetko, Patrizio Lancellotti, Marco Matucci, Theresa McDonagh, Luc A. Pierard, Pedro T. Trindade, Maurizio Zompatori and Marius Hoeper.Eur Respir J2015; 46: 903–975. Eur
Respir J European Respiratory Journal. 2015;46(6):1855–6.](https://image.slidesharecdn.com/c6c932b9-6d38-4693-9422-991899a2745f-161007133058/75/Presentation-18-2048.jpg)