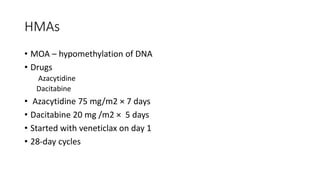
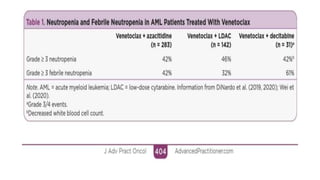
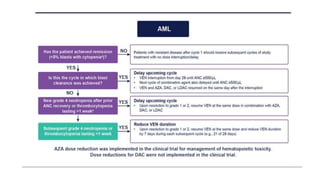
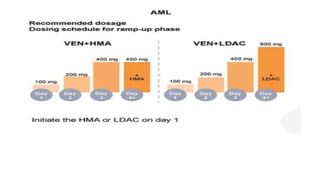
The document details the adverse effects and management of chemotherapy agents, particularly cytarabine, doxorubicin, and venetoclax used in treating acute myeloid leukemia (AML). It addresses specific toxicities like myelosuppression, gastrointestinal issues, and neurotoxicity, highlighting the grading of neutropenia risk and the protocols for managing complications. Additionally, it emphasizes the importance of monitoring and adjusting treatment based on patient response and toxicity levels.