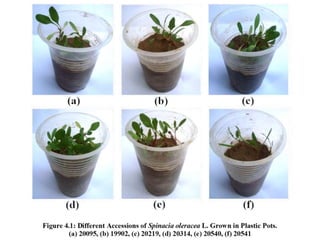
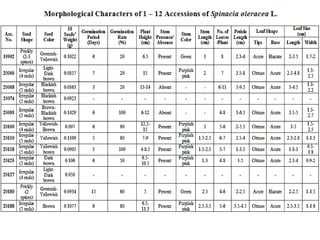
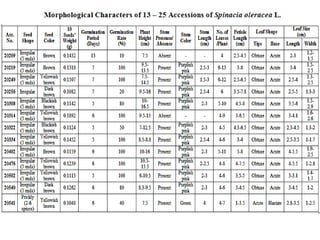
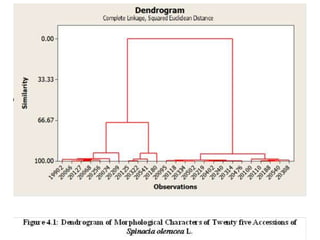
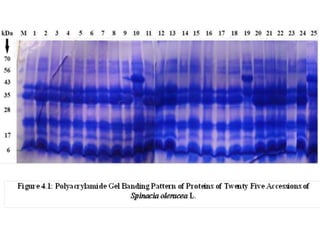
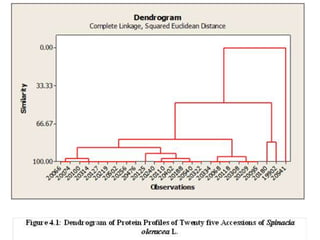
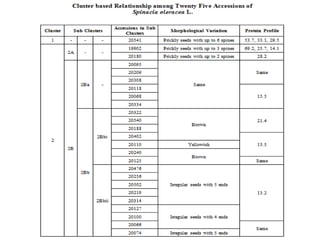
This document summarizes a study assessing genetic diversity in 25 spinach accessions using SDS-PAGE and morphological analysis. Morphological analysis found variation in seed shape, color, stem color, and leaf tips. SDS-PAGE identified 27 total protein bands, with 20 being polymorphic. Three accessions from different locations (20541 from Peshawar, 19902 from AVRDC, and 20180 from Lahore) showed significant genetic differences based on presence of distinct protein bands. While genetic differences were linked to morphological variations, no clear linkage was found between diversity and geographic origin of the accessions. The study concluded spinach accessions show genetic diversity that could benefit future breeding programs.