Embed presentation
Download to read offline
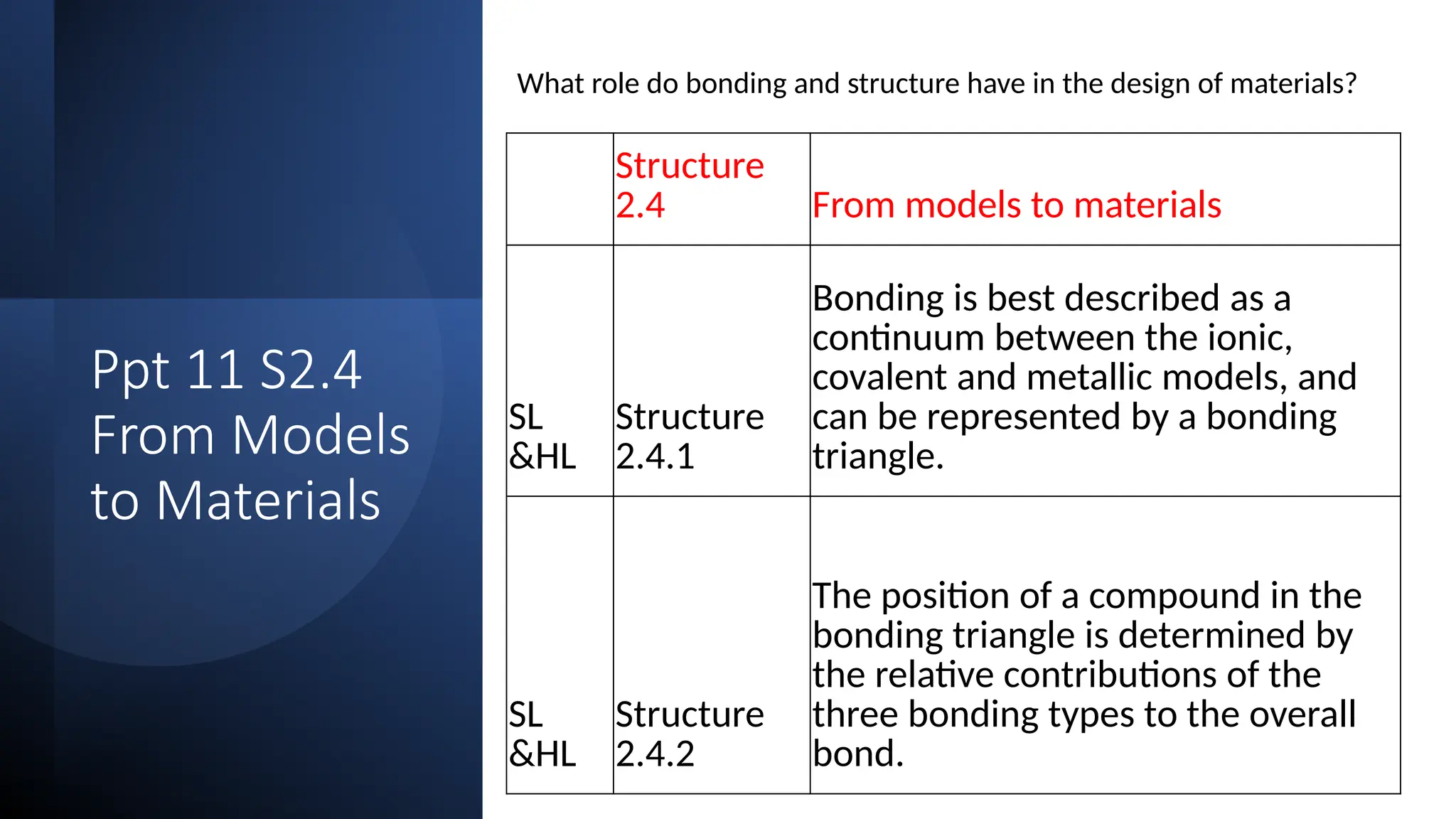
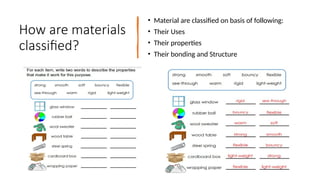
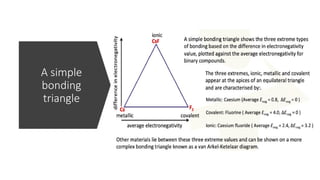

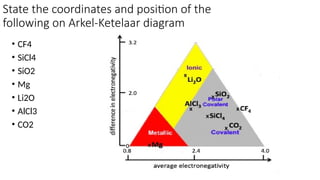

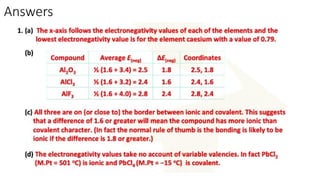

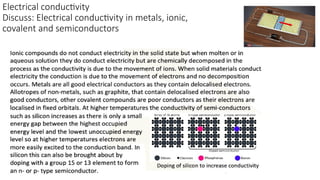
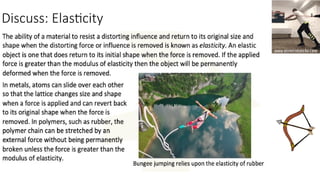



The document discusses the continuum of bonding types—ionic, covalent, and metallic—and how they can be represented in a bonding triangle. It covers the classification of materials based on their uses, properties, and structure, referencing a Van Arkel-Ketelaar diagram to position various compounds. Additionally, it addresses topics like electrical conductivity, elasticity, brittleness, and ductility in the context of different material types.












