Embed presentation
Download to read offline

![pH
• A logarithmic
measure the
concentration of
hydrogen ions in an
aqueous solution.
Log base 10
[H+] : Concentration of hydrogen ions
(moldm-3)](https://image.slidesharecdn.com/powerpointconcentrationacid-240417015008-34ca2544/85/POWER-POINT-CONCENTRATION-ACID-pptx-IGCSE-FORM-3-4-320.jpg)

![pH value for alkali
[OH-]: concentration of hydroxide ions (moldm-3)](https://image.slidesharecdn.com/powerpointconcentrationacid-240417015008-34ca2544/85/POWER-POINT-CONCENTRATION-ACID-pptx-IGCSE-FORM-3-7-320.jpg)

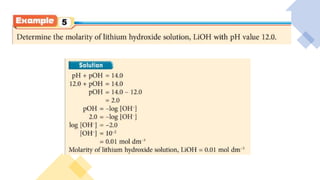
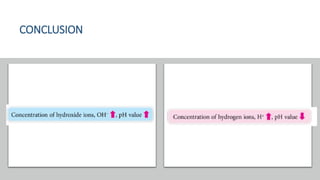

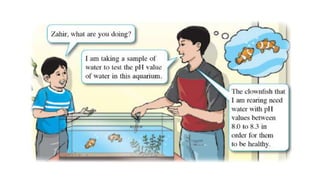
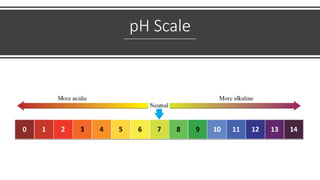
pH is a logarithmic measure of the concentration of hydrogen ions in a solution, with lower pH indicating higher acidity. The pH scale runs from 0 to 14, with 7 being neutral, below 7 acidic, and above 7 alkaline. pH is determined by taking the negative logarithm of the molar concentration of hydrogen ions.



![pH
• A logarithmic
measure the
concentration of
hydrogen ions in an
aqueous solution.
Log base 10
[H+] : Concentration of hydrogen ions
(moldm-3)](https://image.slidesharecdn.com/powerpointconcentrationacid-240417015008-34ca2544/85/POWER-POINT-CONCENTRATION-ACID-pptx-IGCSE-FORM-3-4-320.jpg)


![pH value for alkali
[OH-]: concentration of hydroxide ions (moldm-3)](https://image.slidesharecdn.com/powerpointconcentrationacid-240417015008-34ca2544/85/POWER-POINT-CONCENTRATION-ACID-pptx-IGCSE-FORM-3-7-320.jpg)




