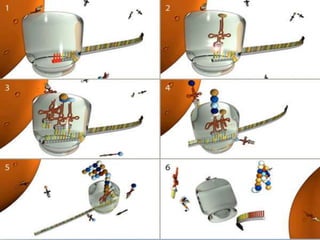
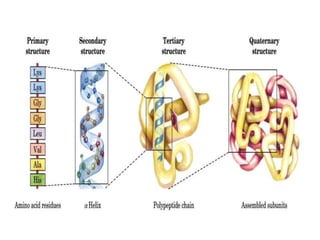
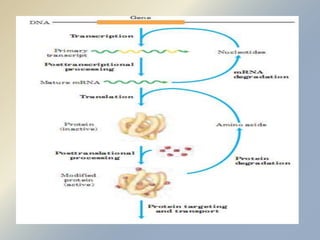
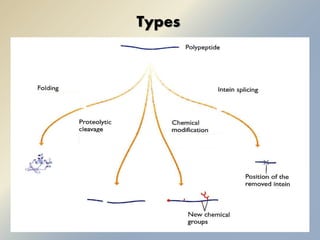
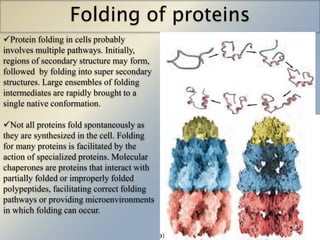
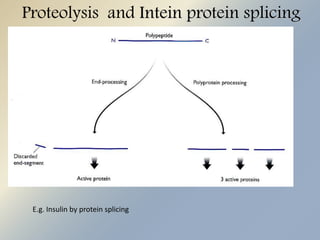
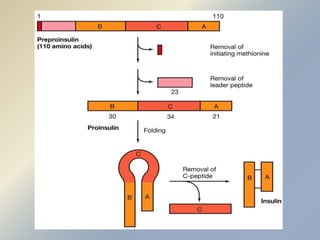
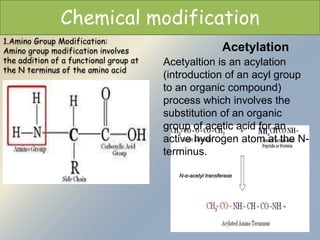
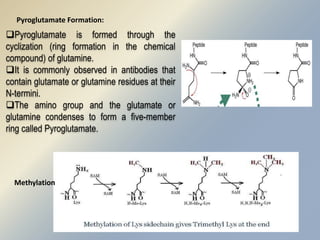
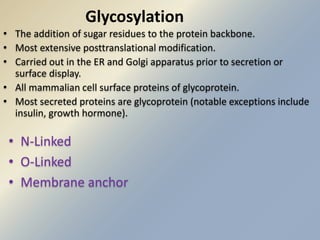
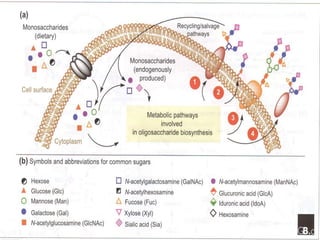
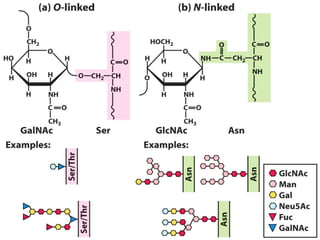
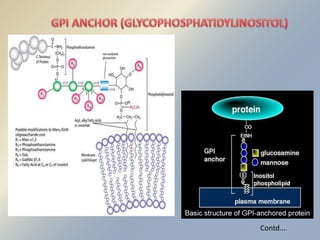
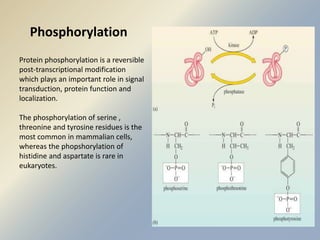
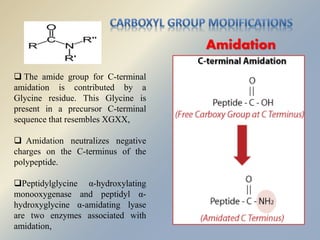
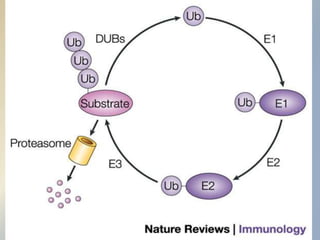
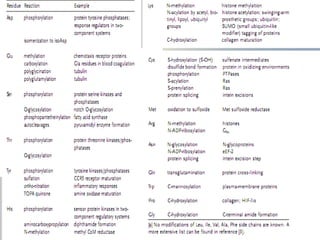
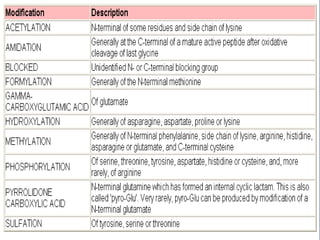
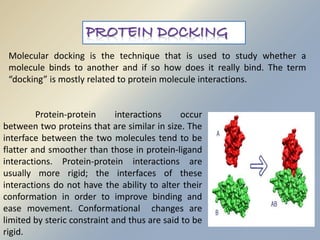
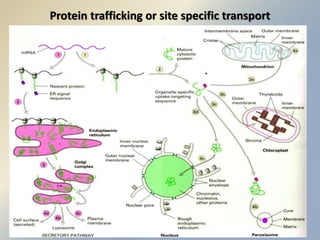
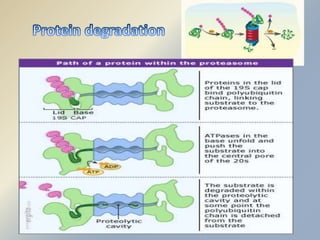
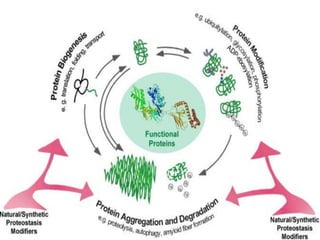
Posttranslational modifications occur in different locations in the cell and serve various purposes. The main locations for modification include the nucleus, lysosome, mitochondria, Golgi, ER, cytosol, ribosome, plasma membrane, extracellular fluid, and extracellular matrix. Common types of modifications include acetylation, phosphorylation, glycosylation, amidation, and ubiquitination. These modifications influence protein stability, activity, localization, and signaling interactions.