Embed presentation
Download to read offline







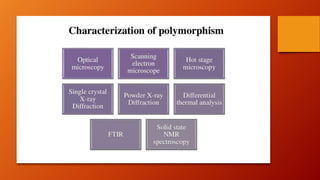
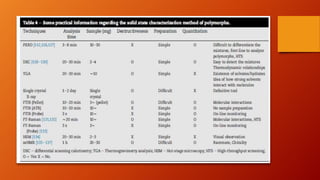

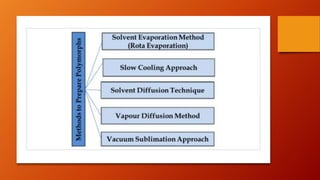

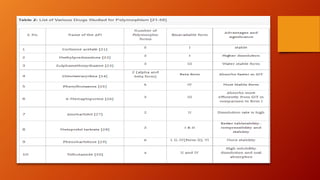



Polymorphism refers to different crystalline forms of a drug substance that differ in physical properties. Polymorphism often occurs unintentionally during drug development and manufacturing. About half of drug candidates fail clinical trials due to efficacy, safety or solubility issues related to polymorphism. Proper evaluation of a drug's polymorphic forms and their stability is important to ensure the drug's effectiveness and safety.

















