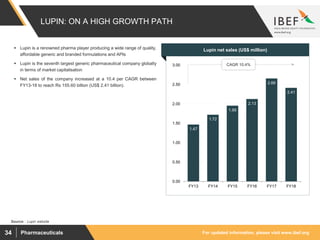
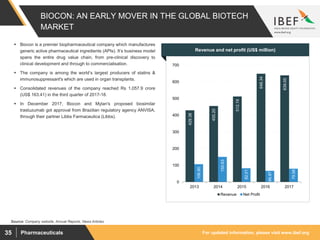
Indian pharmaceutical sector supplies over 50% of global demand for various vaccines and generic drugs. The market is expected to grow at a CAGR of 22.4% until 2020 to reach $55 billion. India accounts for 20% of global exports in generics. Key factors driving growth include rising income levels, increased healthcare access and greater penetration of health insurance. Indian companies are raising R&D spending and entering new markets to sustain growth in the face of increasing competition from global players.