The document outlines the processes of calibration, qualification, and validation in pharmaceutical quality assurance, emphasizing their importance in ensuring accuracy and compliance with industry standards. It details various stages of qualification, including design, installation, operational, and performance qualifications, as well as the significance of routine calibration to maintain measurement integrity. Validation is described as a systematic process to ensure systems consistently achieve desired results, requiring thorough documentation and a multidisciplinary approach for effective execution.


![TABLE OF CONTENTS
Calibration
Qualification
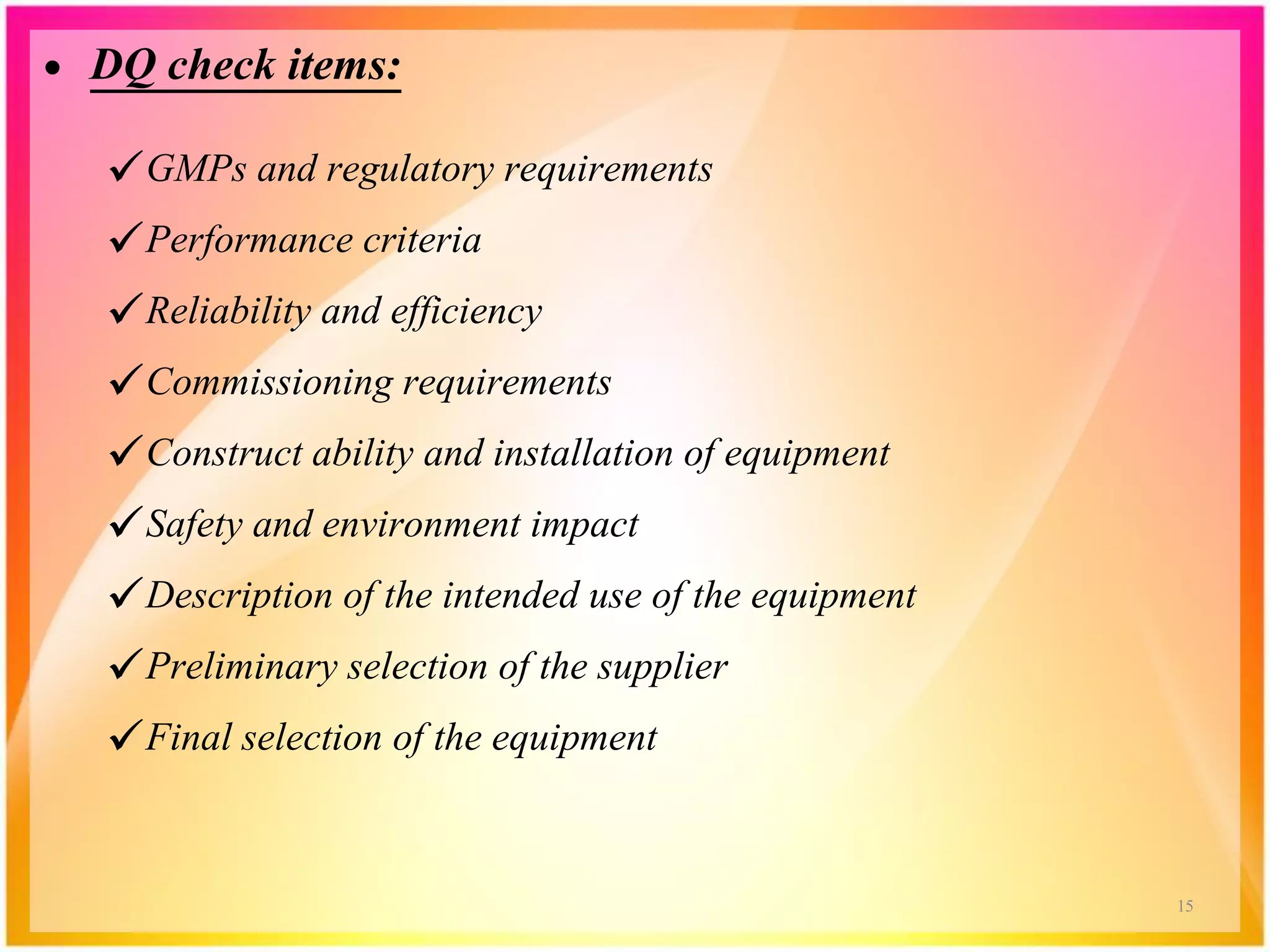
o Design Qualification [Dq]
o Installation Qualification [Iq]
o Operational Qualification [Oq]
o Performance Qualification [Pq]
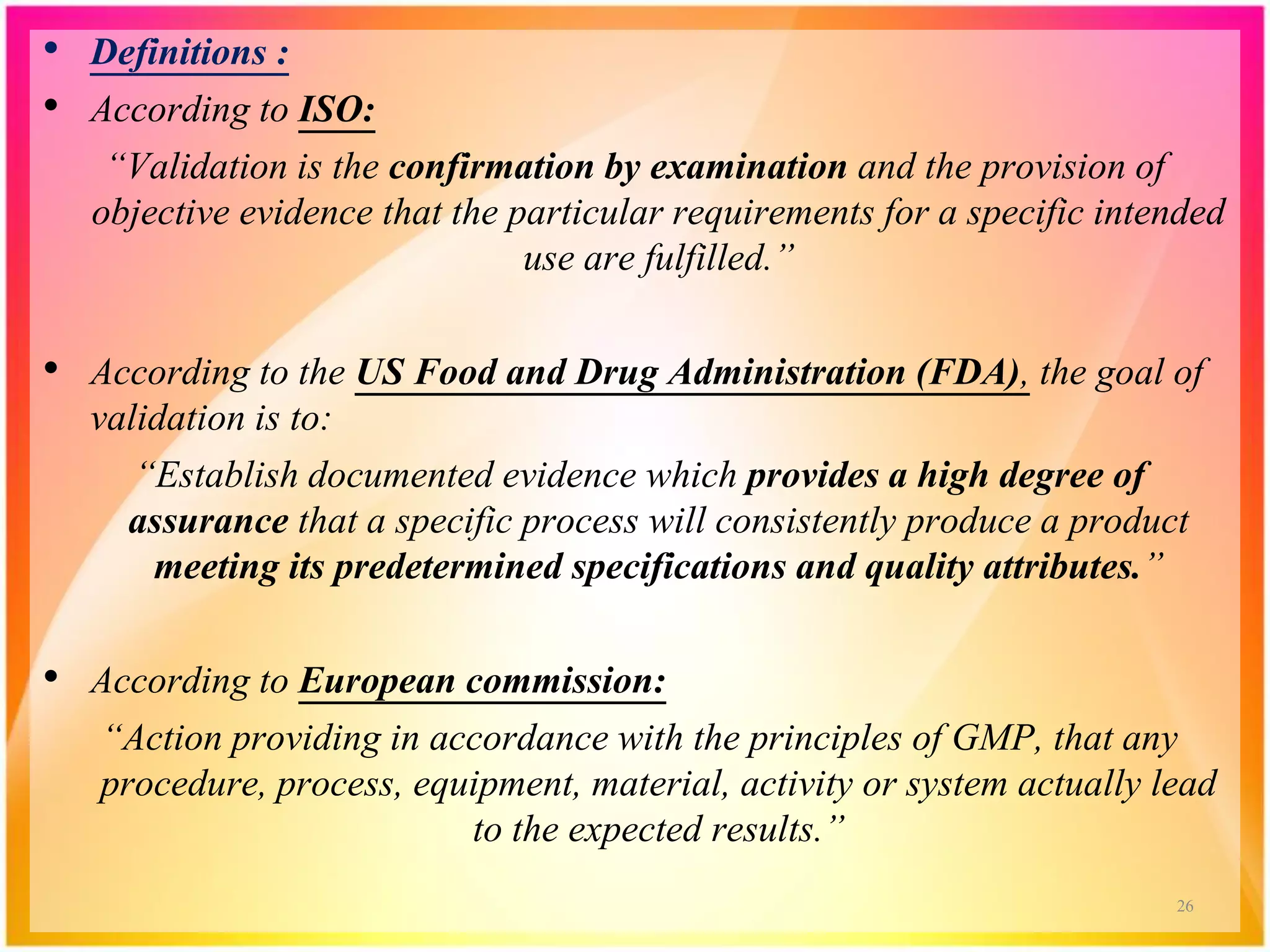
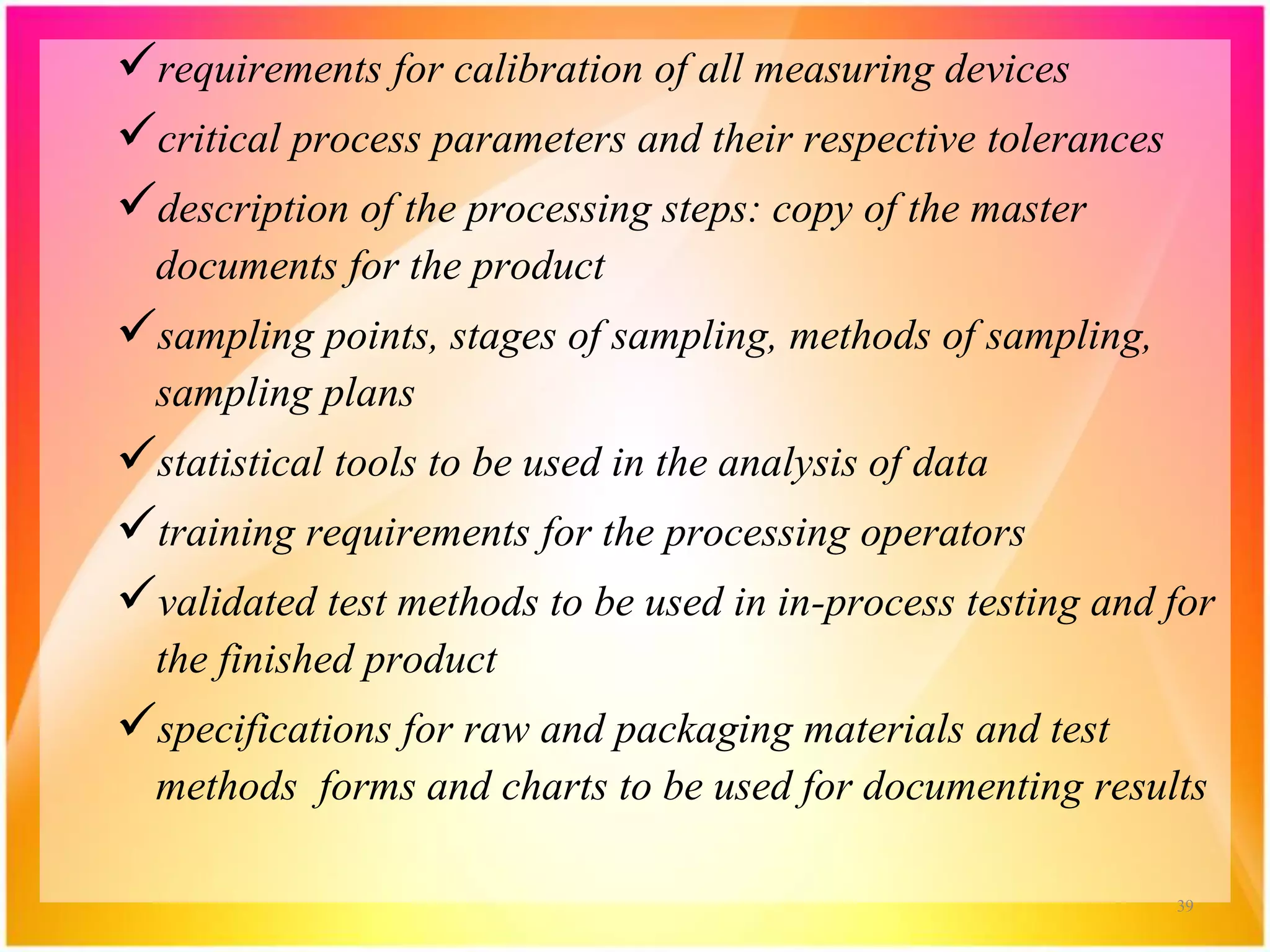
Validation
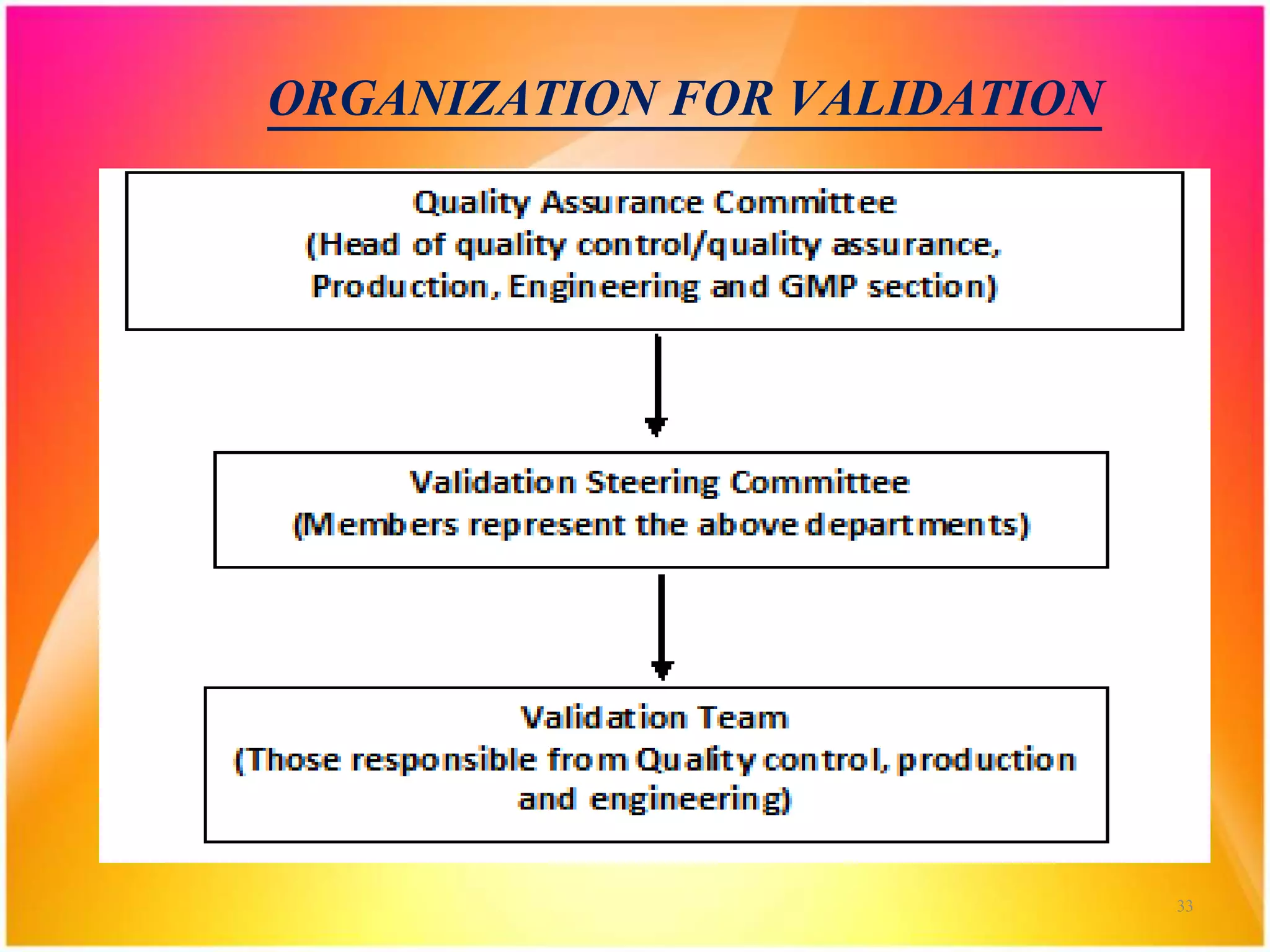
Organization For Validation
Validation Master Plan
Validation Protocol
3](https://image.slidesharecdn.com/princy-171023032007/75/PHARMACEUTICAL-CALIBRATION-QUALIFICATION-AND-VALIDATION-AN-INTRODUCTION-3-2048.jpg)