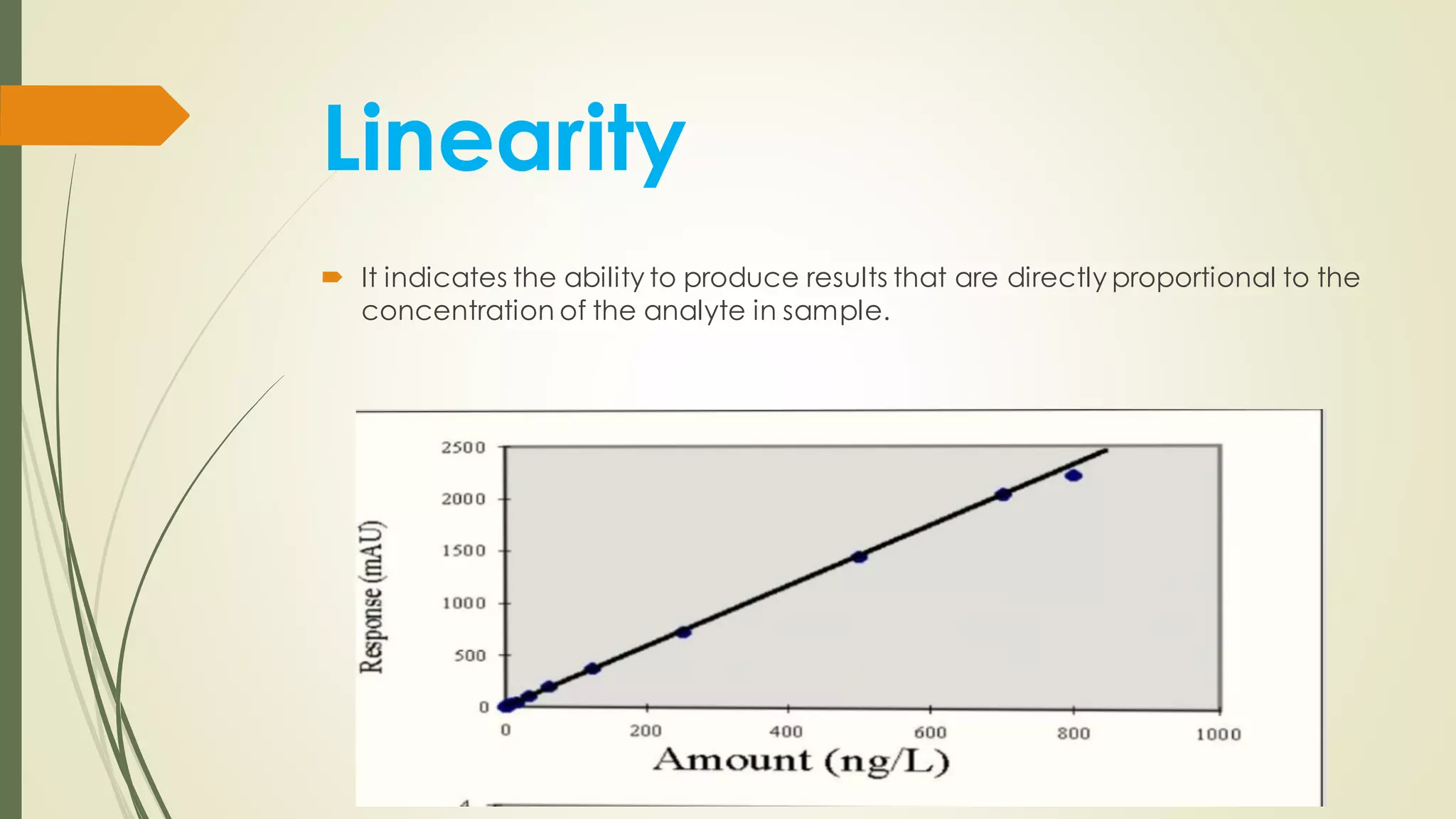
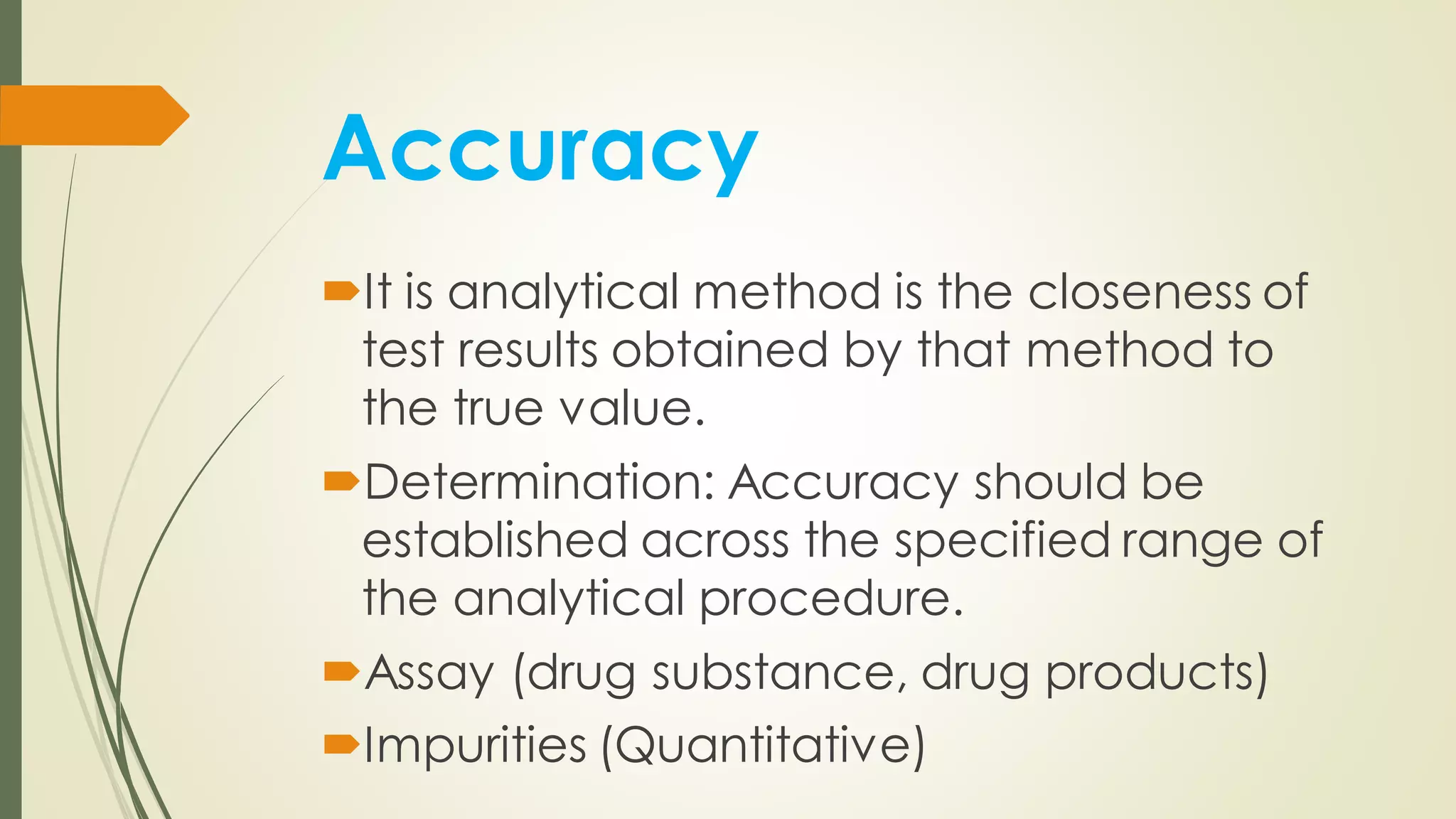
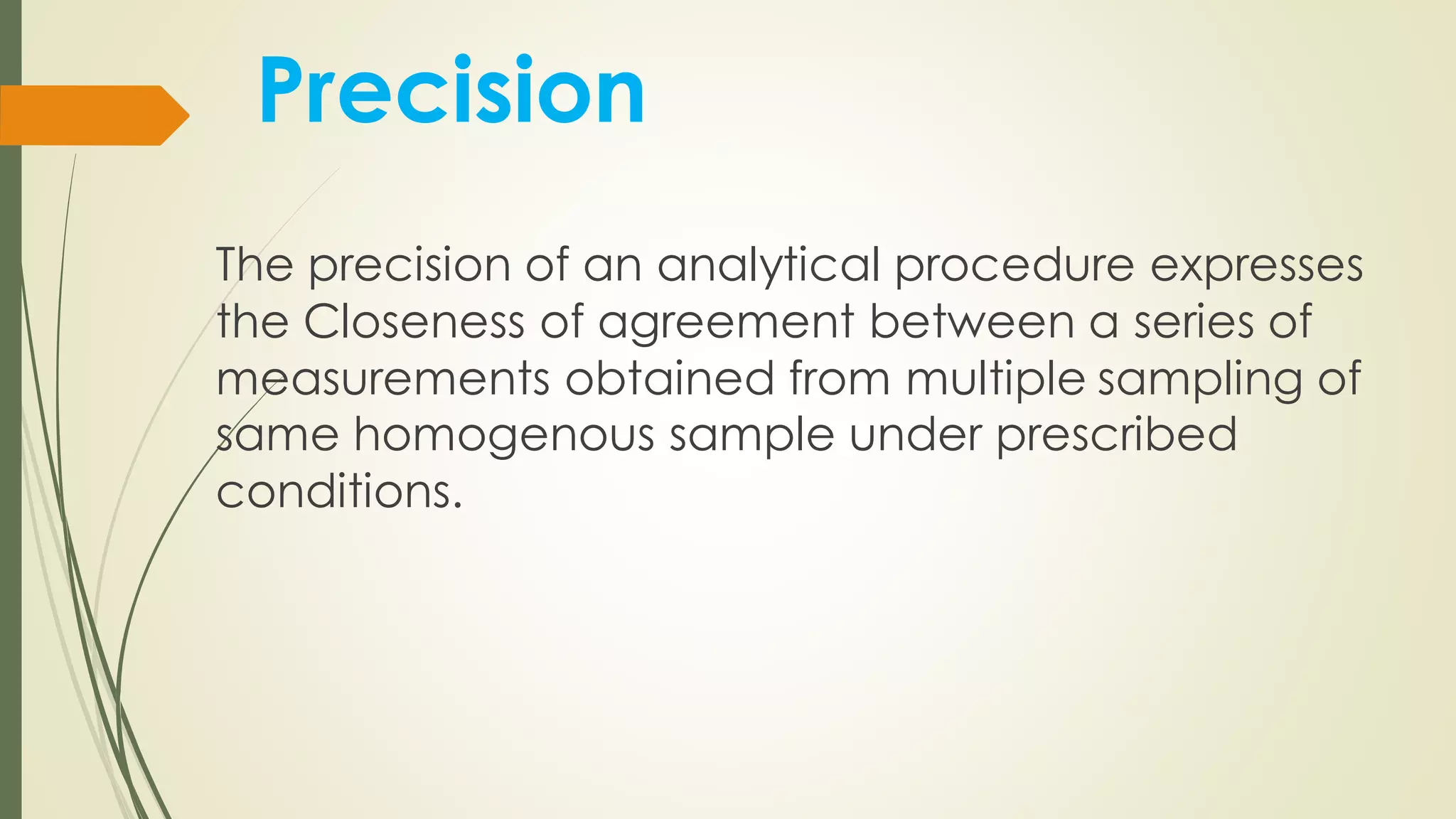
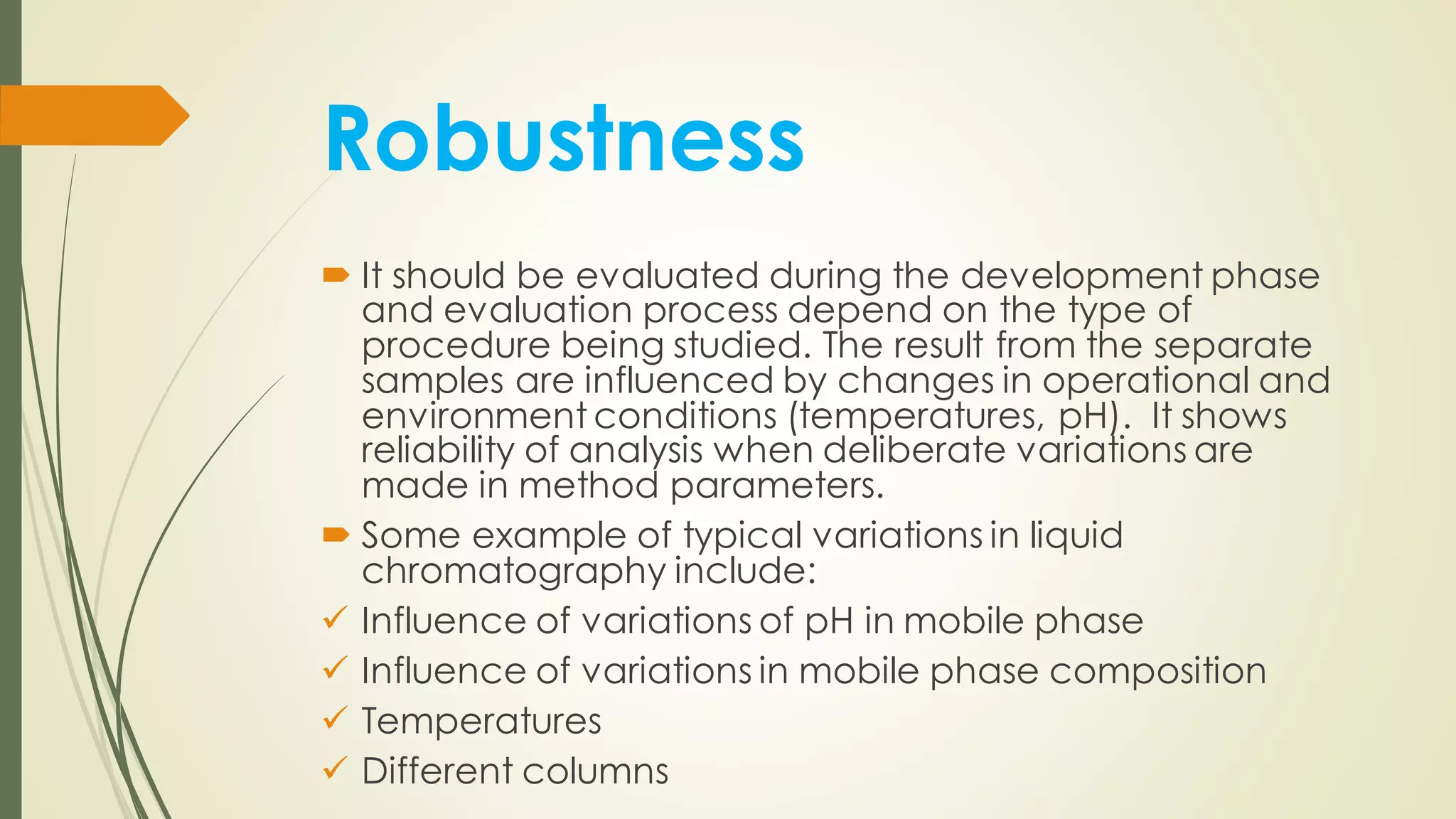
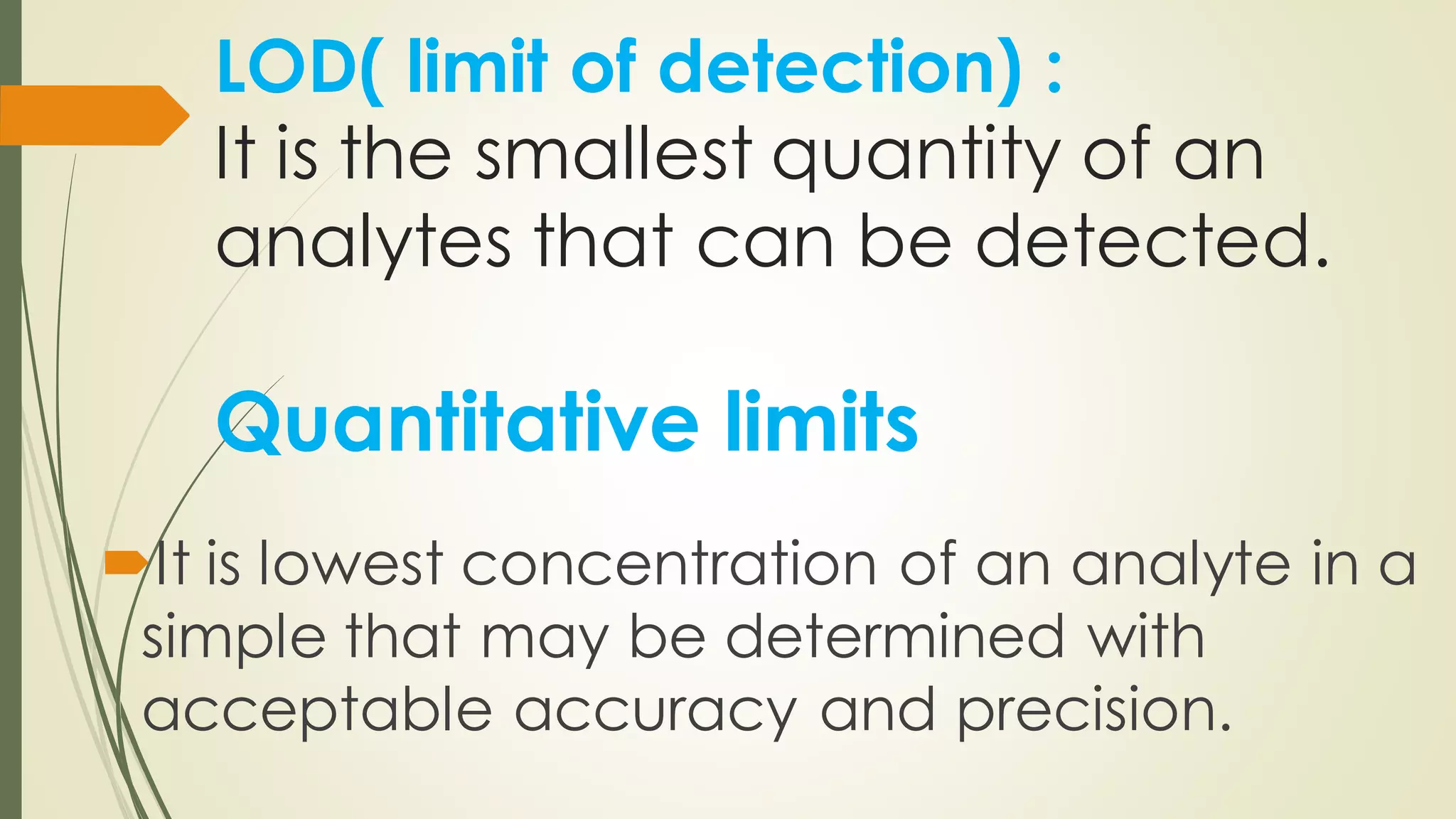
The document outlines the general principles of analytical method validation, emphasizing its importance in ensuring the compliance and quality of pharmaceutical products through systematic studies. It discusses the types of analytical procedures that require validation, the characteristics to be assessed, and the need for revalidation when changes occur. Key validation characteristics include specificity, accuracy, precision, and robustness, which together ensure the reliability of analytical methods in determining the quality and purity of drug substances.