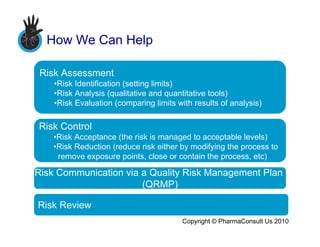
This document discusses managing risks related to cross contamination and contamination in the pharmaceutical manufacturing process. It provides an overview of how regulators are concerned with cross contamination between drugs and outlines the science and risk-based approach taken in ISPE's Risk MaPP Baseline Guide. The document then describes how risk assessment involves identifying, analyzing, and evaluating risks, and how risk control involves accepting, reducing, or removing risks. It also discusses communicating risks via a quality risk management plan. Finally, it offers pharmaceutical manufacturing services and tools for assessing cross contamination risks.