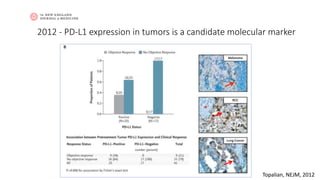
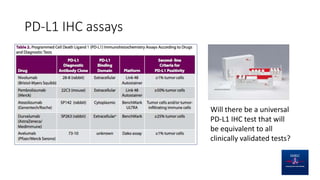
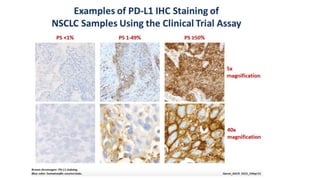
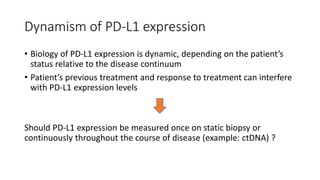
This document discusses PD-L1 testing and its role in immune checkpoint inhibition. It covers the historical context of PD-L1's role in immune escape and checkpoints. It notes that PD-L1 testing has nuances regarding sample type, preanalytic conditions, assay methods, scoring, and the dynamic nature of PD-L1 expression over time and with treatment. Issues include tumor heterogeneity, the interval between biopsy and treatment, and how previous treatments can affect expression levels.