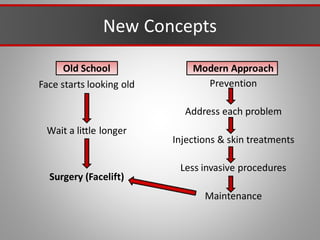
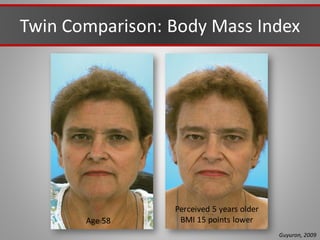
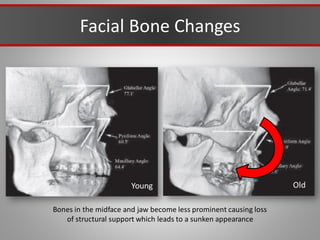
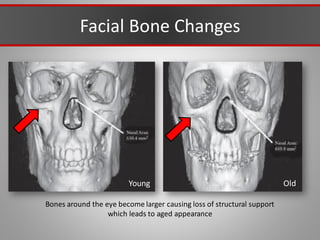
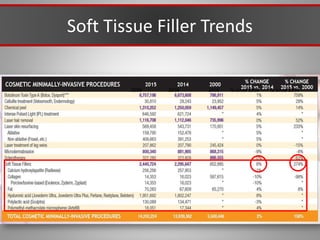
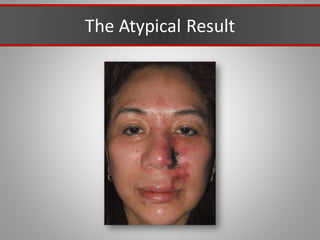
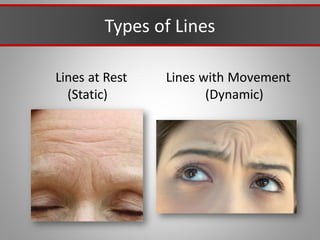
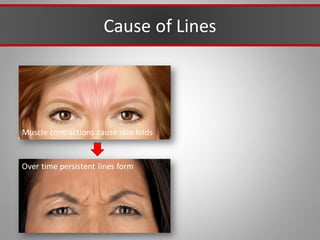
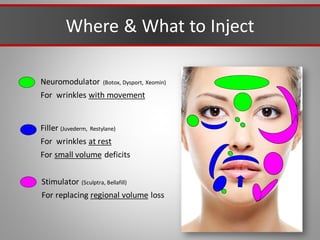
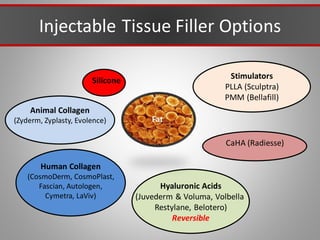
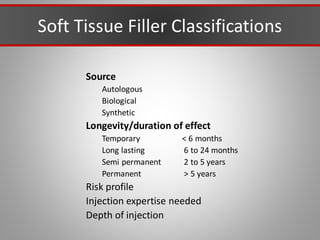
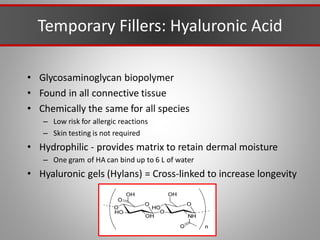
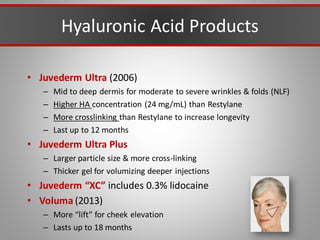
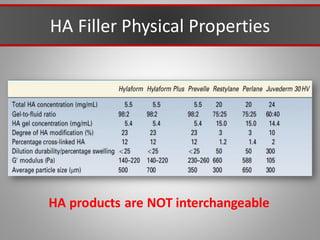
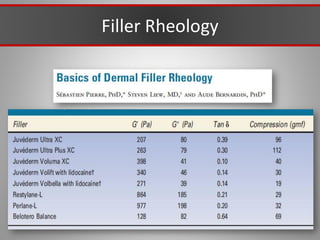
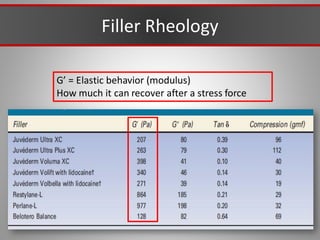
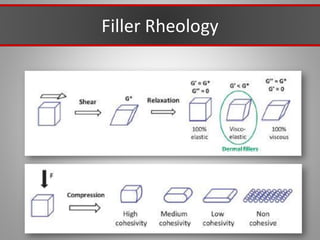
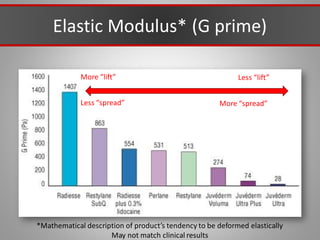
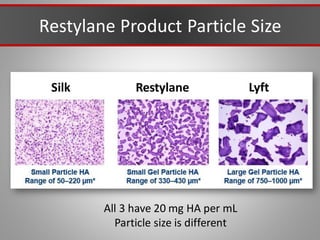
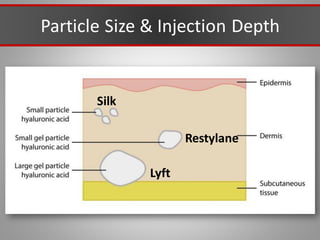
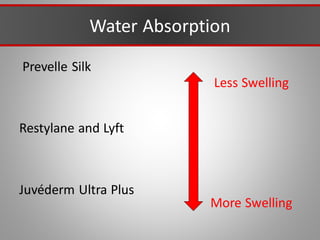
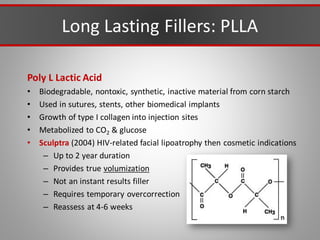
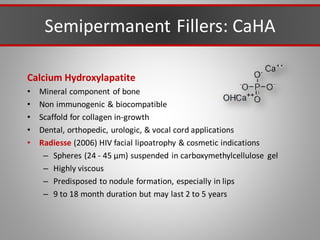
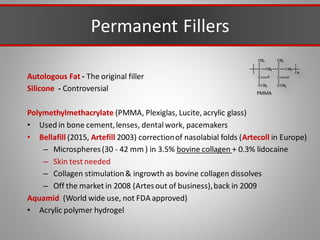
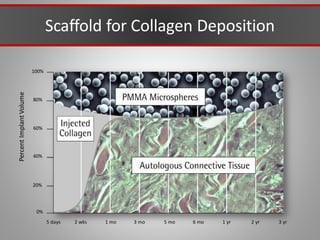
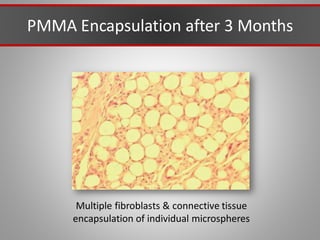
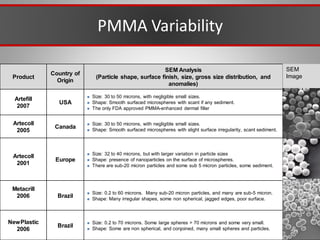
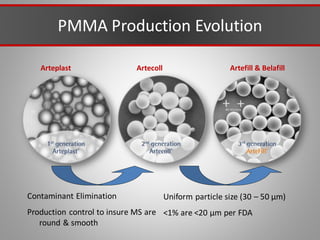
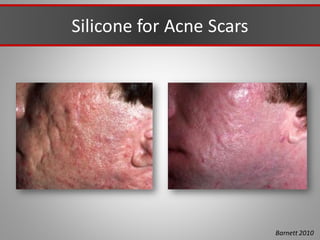
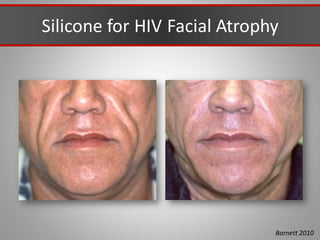
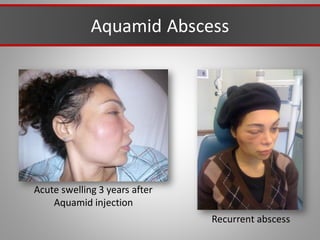
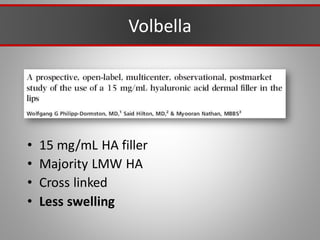
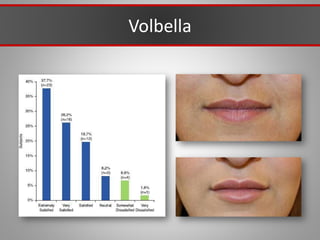
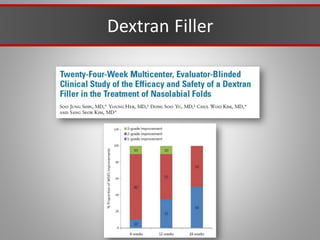
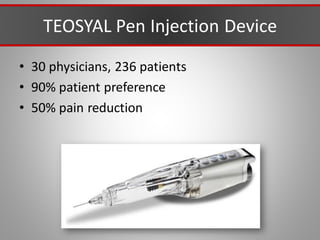
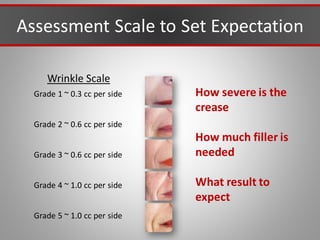
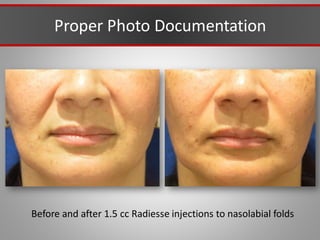
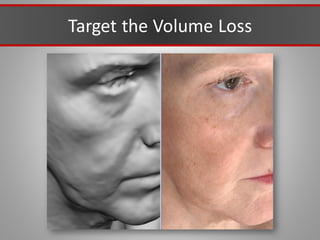
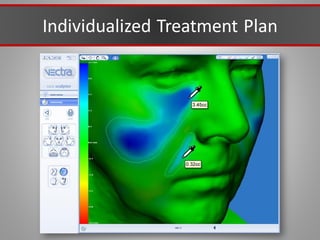
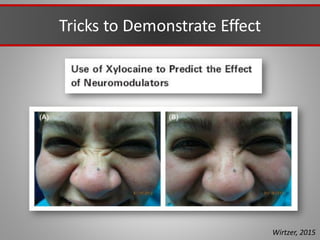
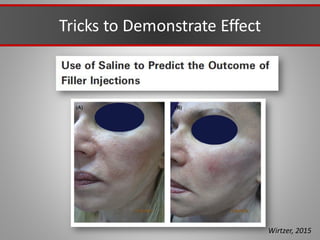
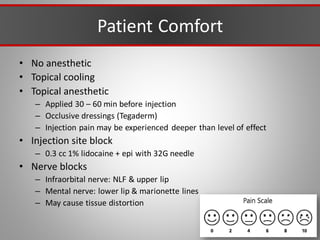
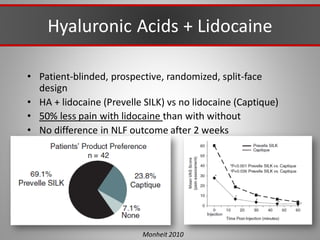
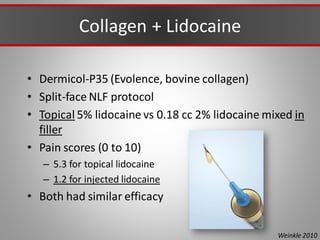
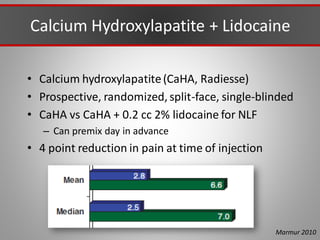
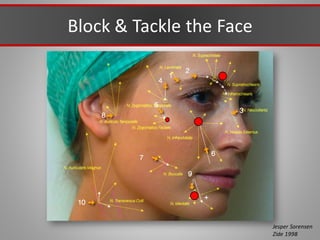
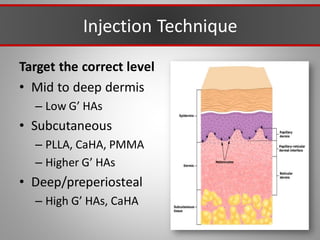
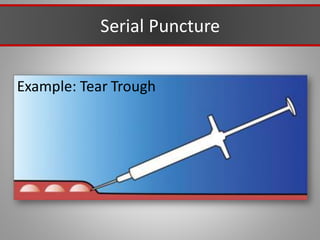
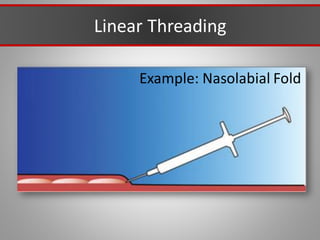
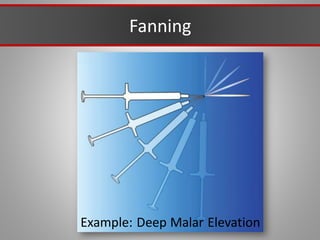
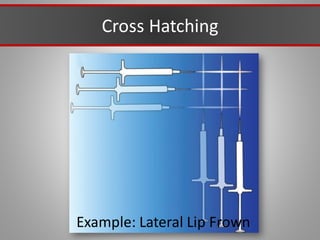
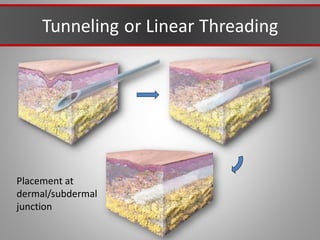
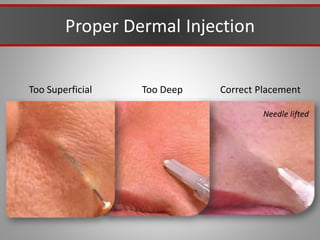
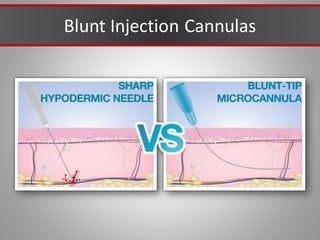
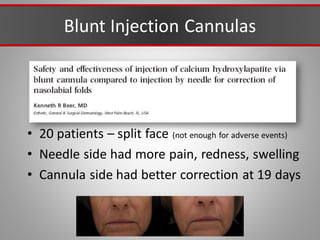
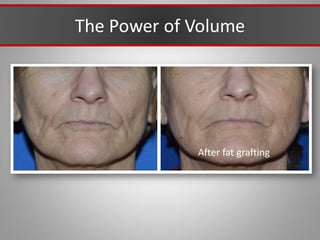
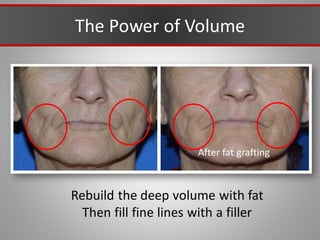
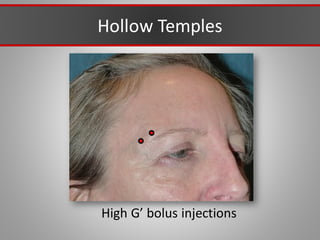
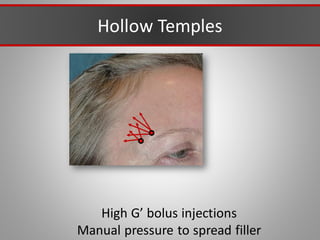
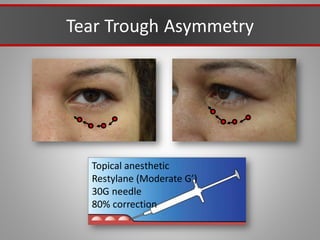
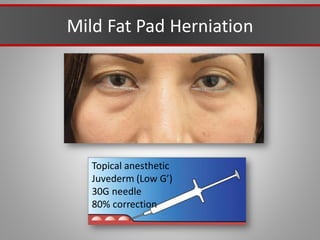
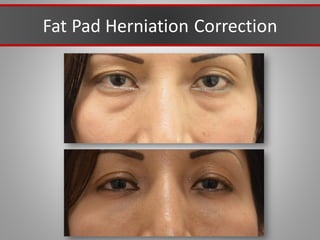
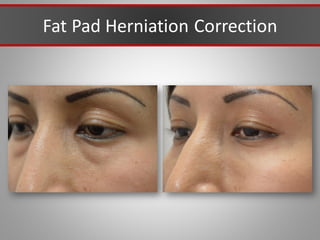
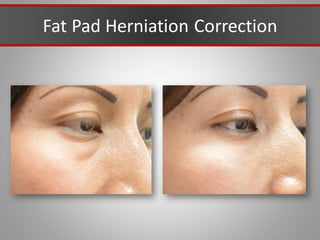
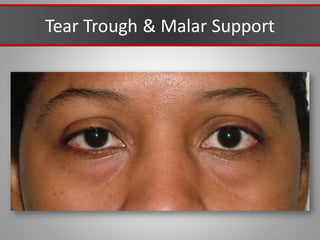
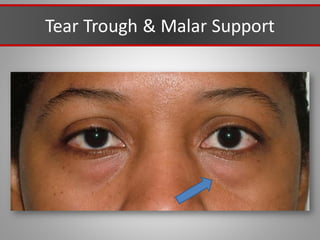
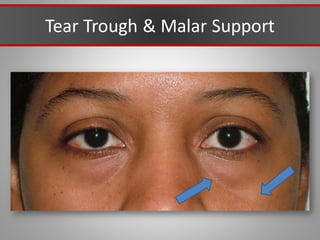
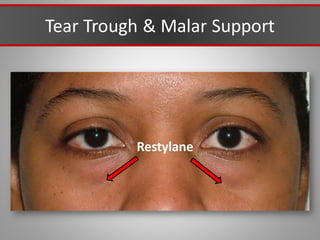
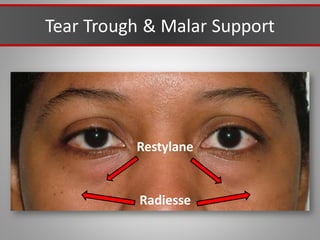
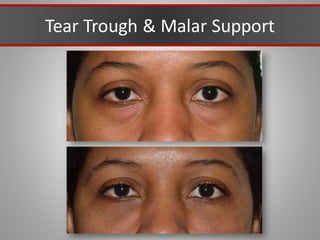
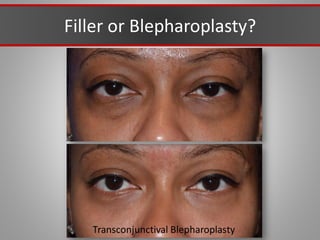
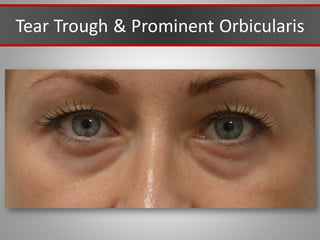
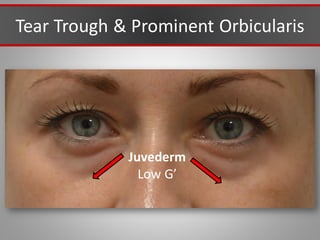
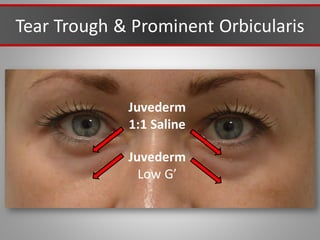
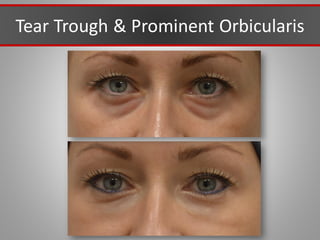
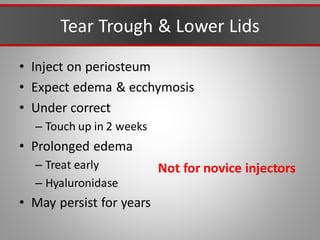
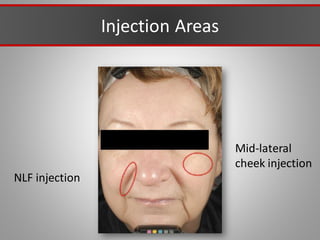
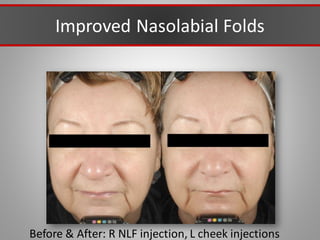
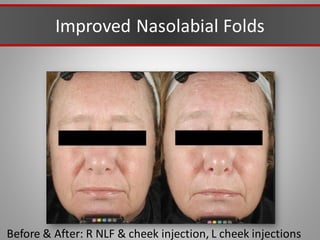
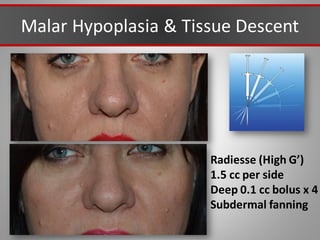
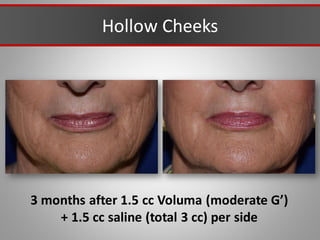
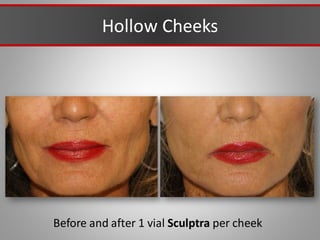
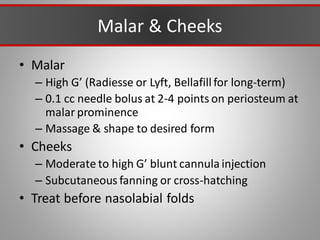
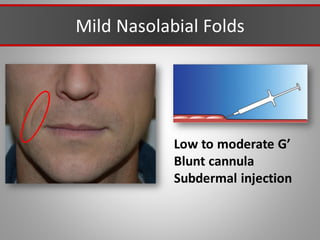
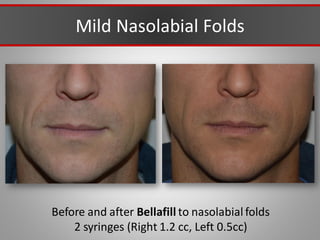
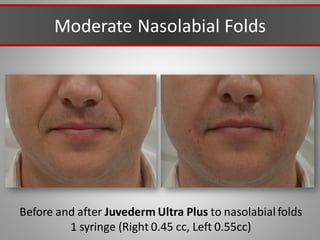
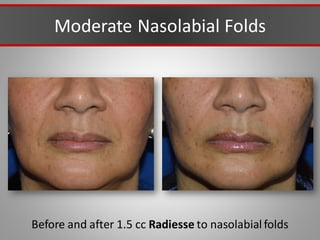
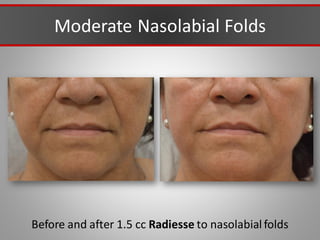
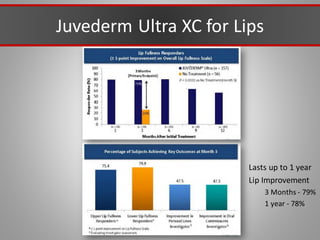
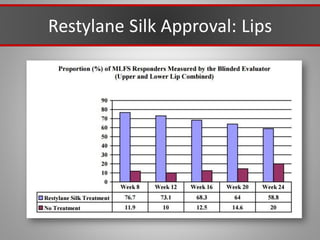
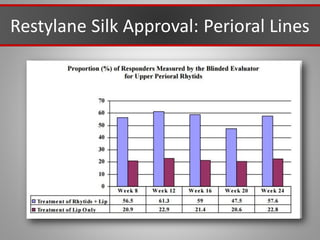
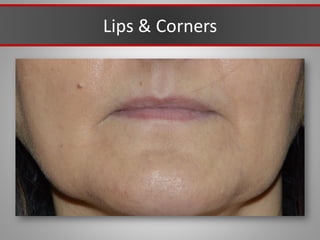
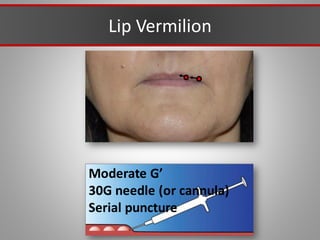
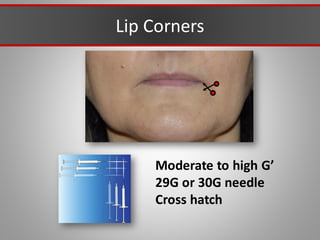
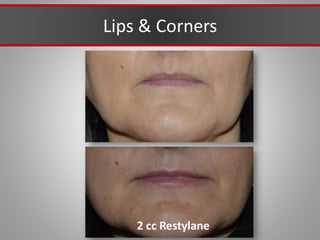
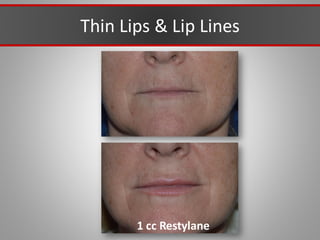
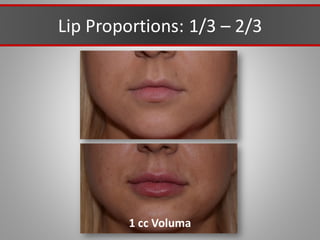
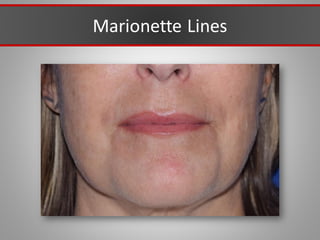
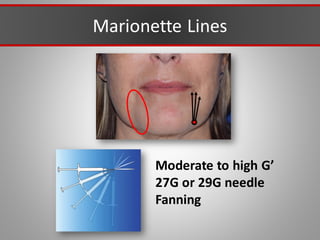
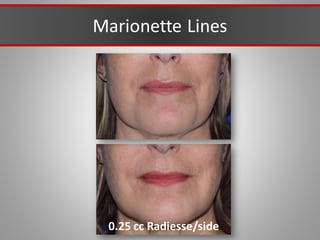
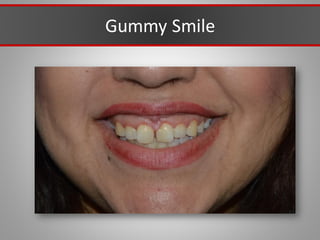
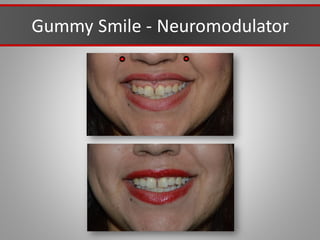
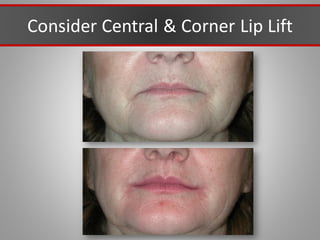
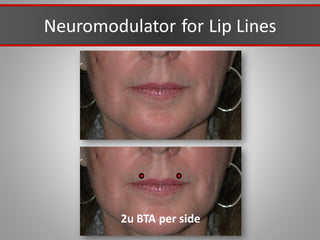
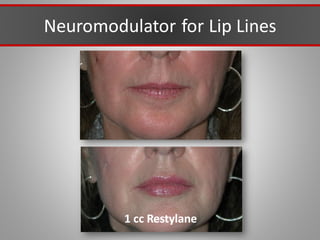
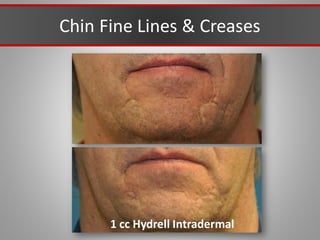
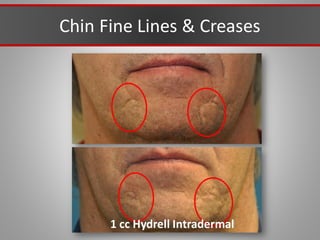
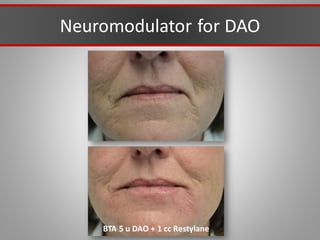
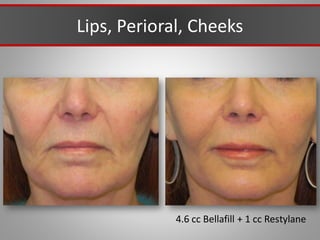
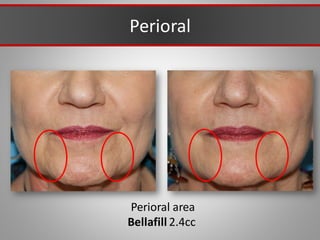
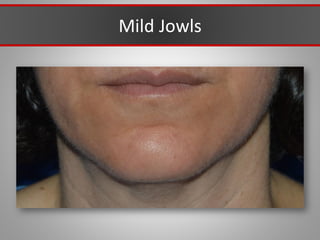
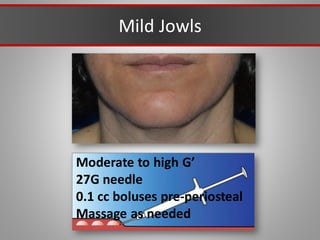
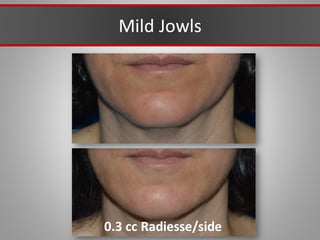
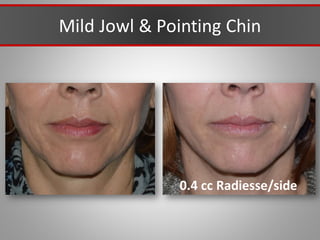
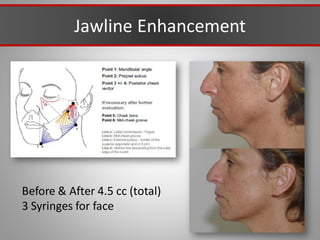
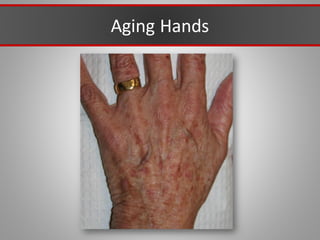
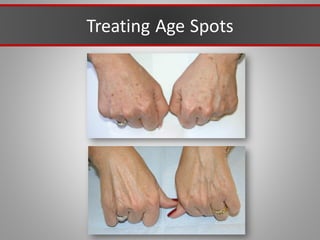
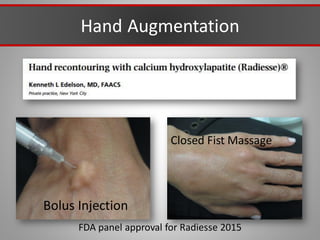
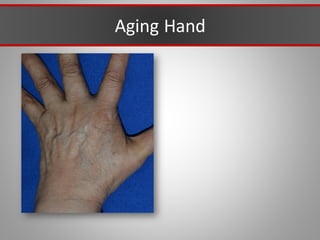
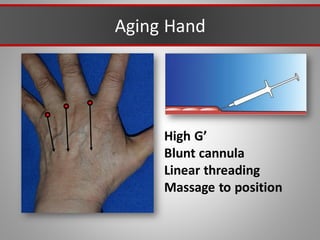
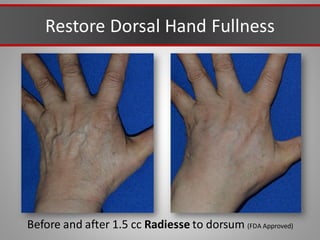
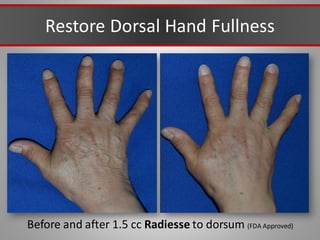
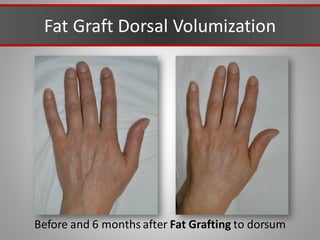
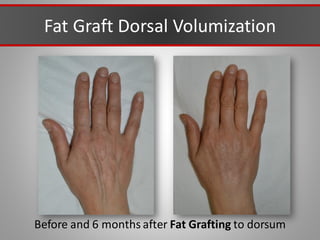
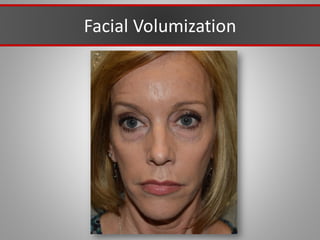
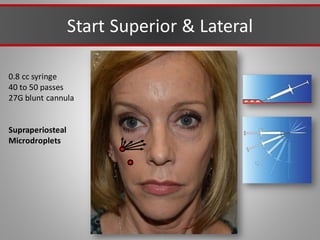
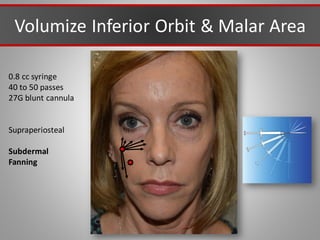
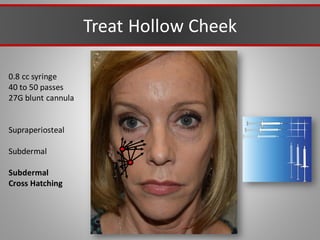
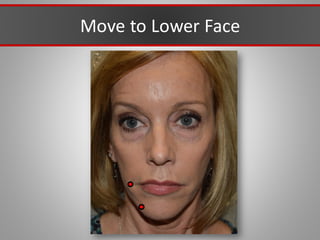
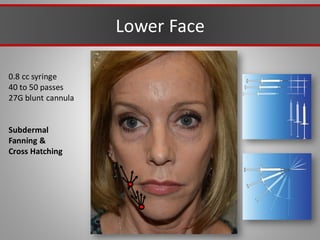
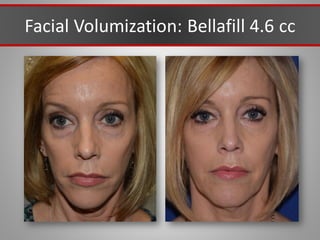
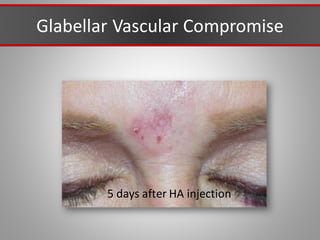
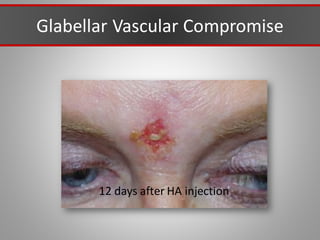
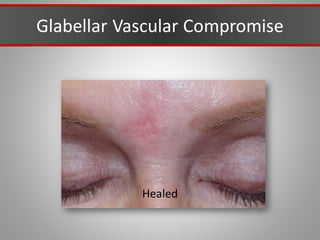
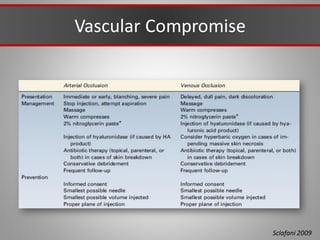
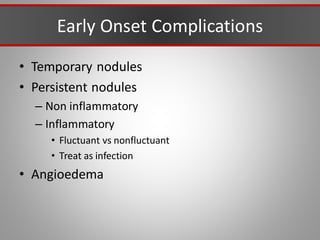
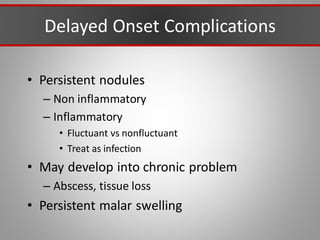
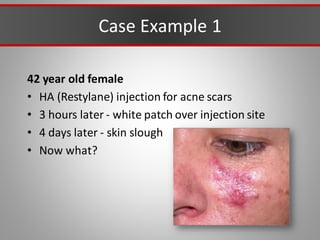
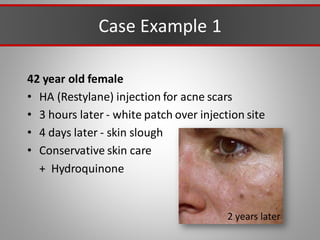
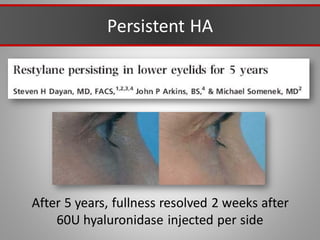
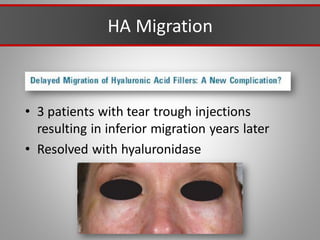
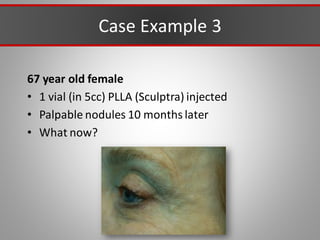
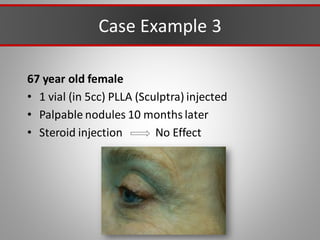
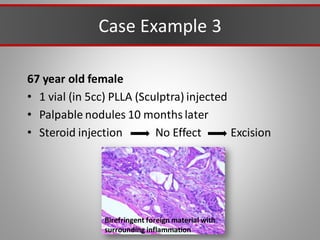
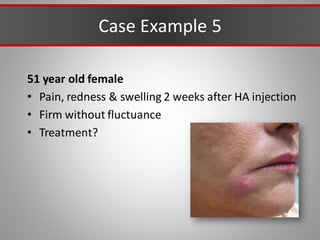
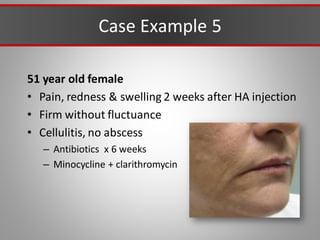
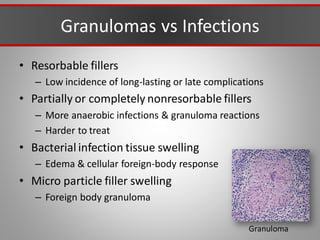
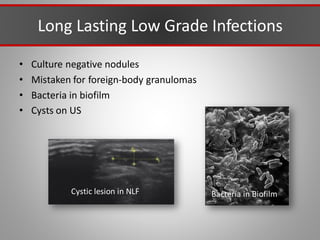
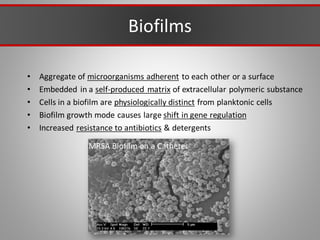
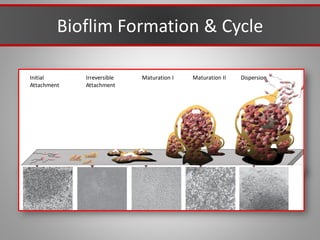
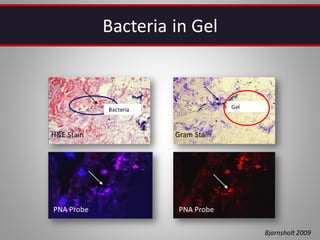
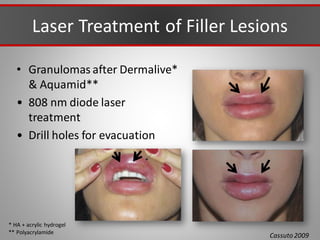
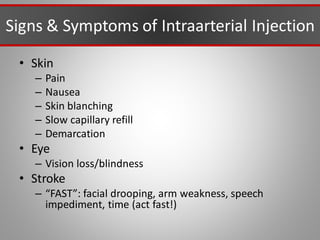
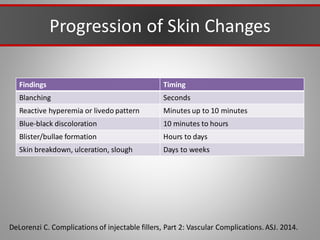
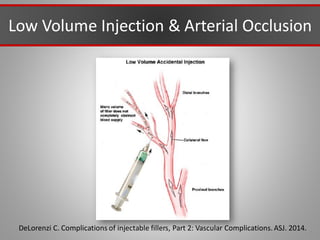
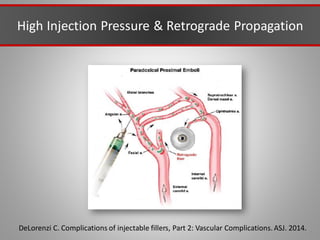
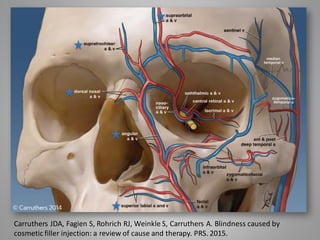
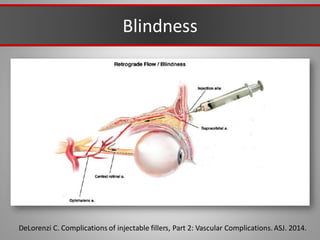
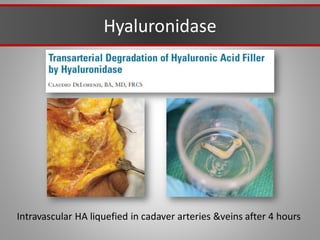
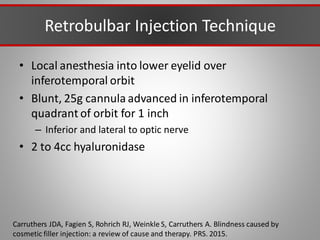
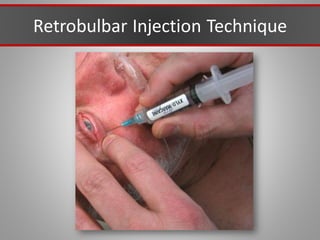
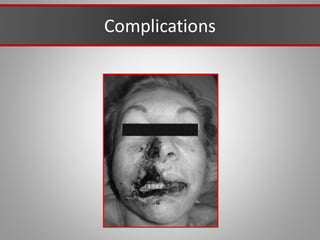
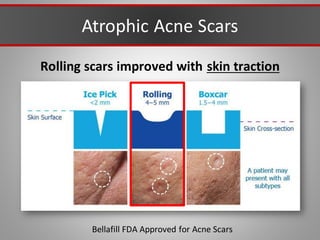
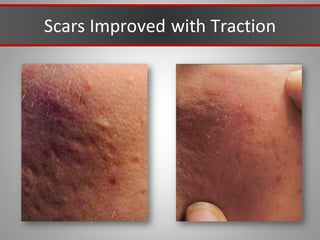
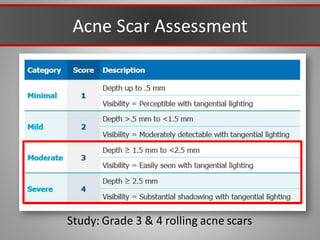
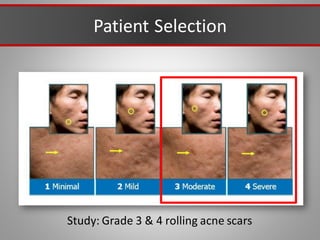
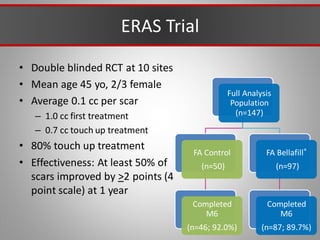
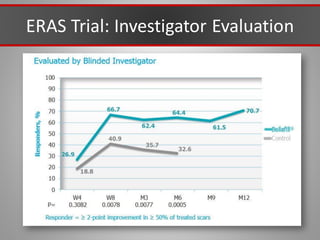
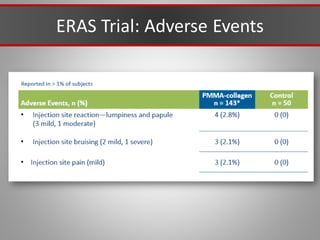
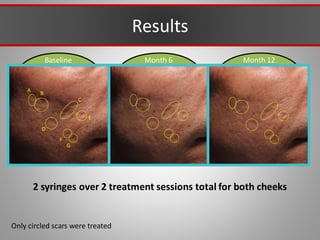
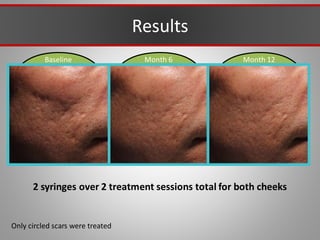
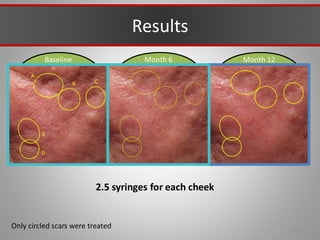
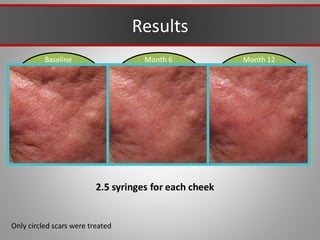
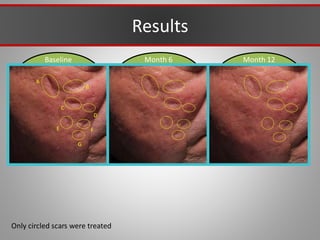
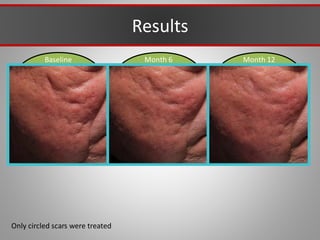
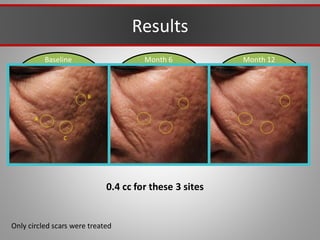
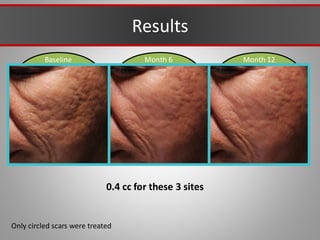
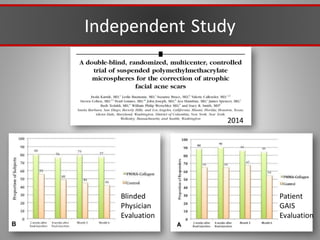
This document discusses injectable soft tissue fillers, including practical applications and considerations. It provides an overview of facial aging assessment and options for addressing volume loss and skin changes. Various filler types and products are compared, including temporary fillers like hyaluronic acid and collagen, long-lasting fillers like PLLA, and semi-permanent or permanent options. Injection sites, potential complications, and regulatory issues are also reviewed.