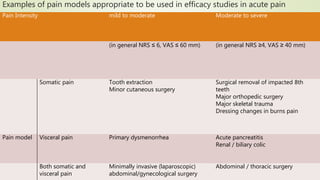
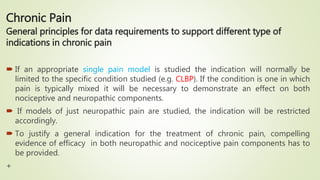
This document provides guidance on clinical development of new pain medications. It discusses general considerations like pharmacokinetic and dose response studies. It recommends study designs for exploring efficacy in acute and chronic pain, including appropriate patient populations, endpoints, and trial duration. Safety evaluation over long term use is also addressed. Special populations like children, elderly, and those with renal or hepatic impairment require tailored study approaches.