Embed presentation
Download to read offline

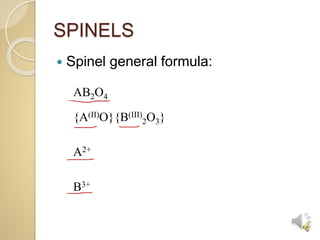



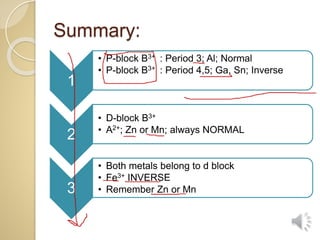
1. This document provides a 3-step technique for determining whether a spinel compound will be normal or inverse based on its chemical formula. It focuses on transition metal spinels commonly found in entrance exams. 2. Step 1 examines the metal cations, step 2 looks at the divalent cation if both are transition metals, and step 3 determines if a transition metal spinel is normal or inverse based on the trivalent cation. 3. The technique establishes general rules like period 3 p-block trivalent cations yielding normal spinels and period 4,5 p-block yielding inverse, and exceptions for certain transition metal combinations.





