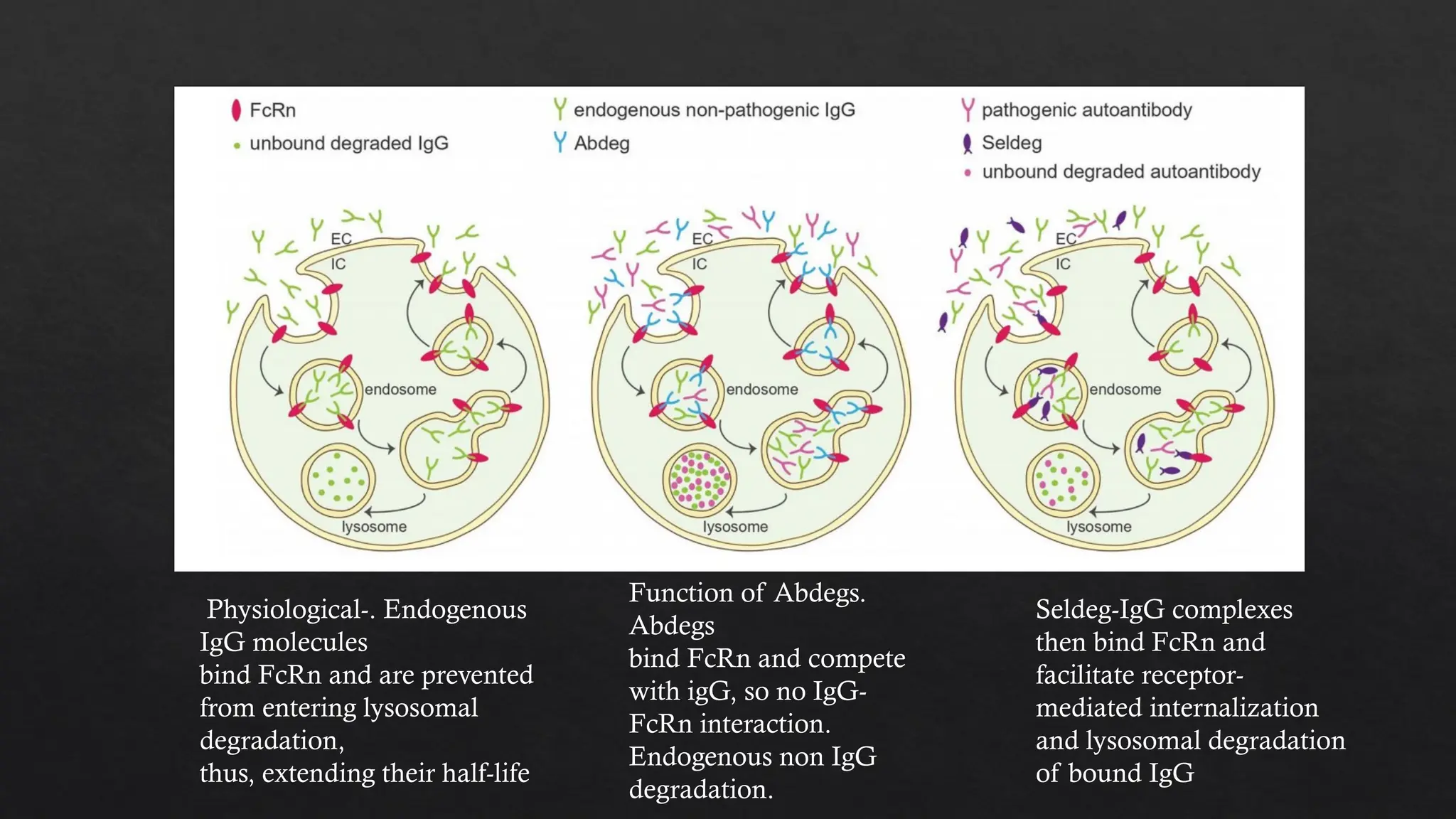
The document discusses neonatal Fc receptor (FCRN) and its critical role in transferring maternal immunity to newborns by recycling IgG antibodies. It highlights recent FCRN-targeted therapies, including FDA-approved drugs like efgartigimod and rozanolixizumab, for conditions such as generalized myasthenia gravis (gMG) and multifocal motor neuropathy (CIDP). The document emphasizes the mechanisms of action of various IgG-engineered therapies and their implications for enhancing IgG half-life and clearance.