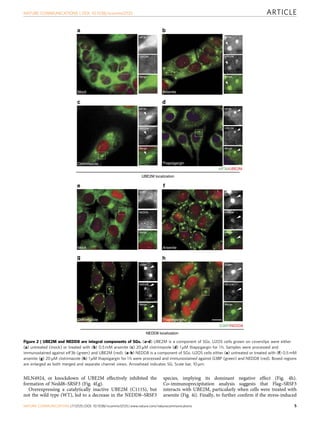
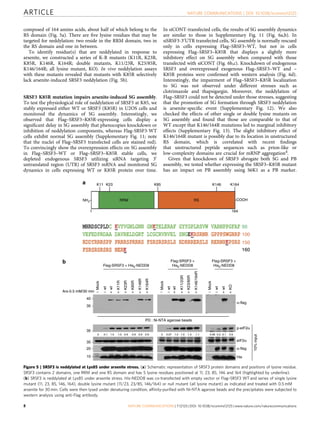
Stress granules (SGs) are cytoplasmic aggregates that form in response to cellular stress and contain translationally stalled messenger ribonucleoproteins (mRNPs). This study shows that neddylation, the covalent attachment of the ubiquitin-like protein NEDD8 to lysine residues on target proteins, promotes SG assembly in response to oxidative stress. Knockdown or inhibition of components of the neddylation pathway impairs stress-induced polysome disassembly and SG assembly. Proteomic analysis identified ribosomal proteins, translation factors, and RNA-binding proteins as potential targets of neddylation in translationally stalled fractions, including the RNA-binding protein SRSF3. SRSF3 is selectively