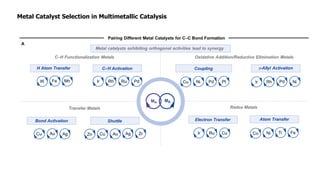
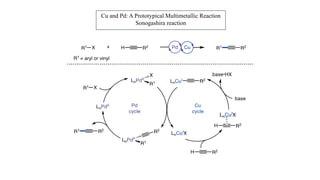
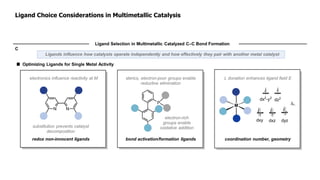
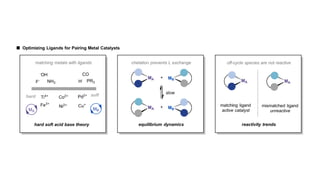
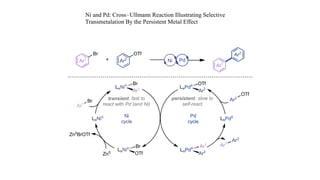
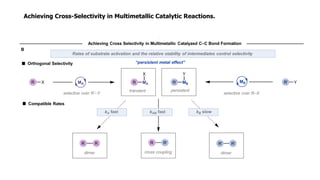
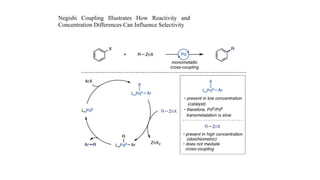
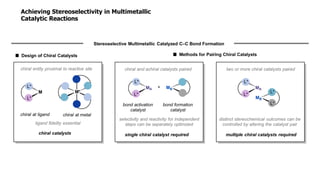
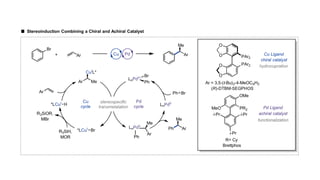
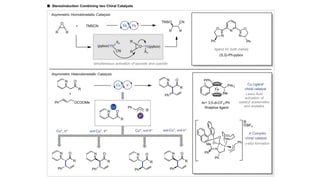
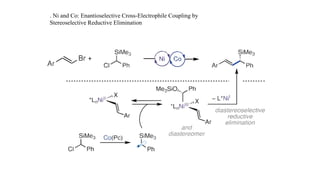
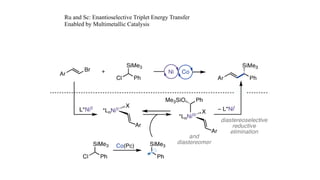
The document discusses principles of multimetallic catalysis for carbon-carbon bond formation. Key considerations in designing multimetallic systems include choosing complementary metals, favoring cross-reactivity between the metals to prevent self-reactivity, and optimizing systems with multiple metals and ligands. Multimetallic reactions can be classified based on how the metals interact, such as one metal transferring electrons to another, one metal activating a substrate that another metal then traps, or metals activating separate substrates that undergo group transfer between the metals.