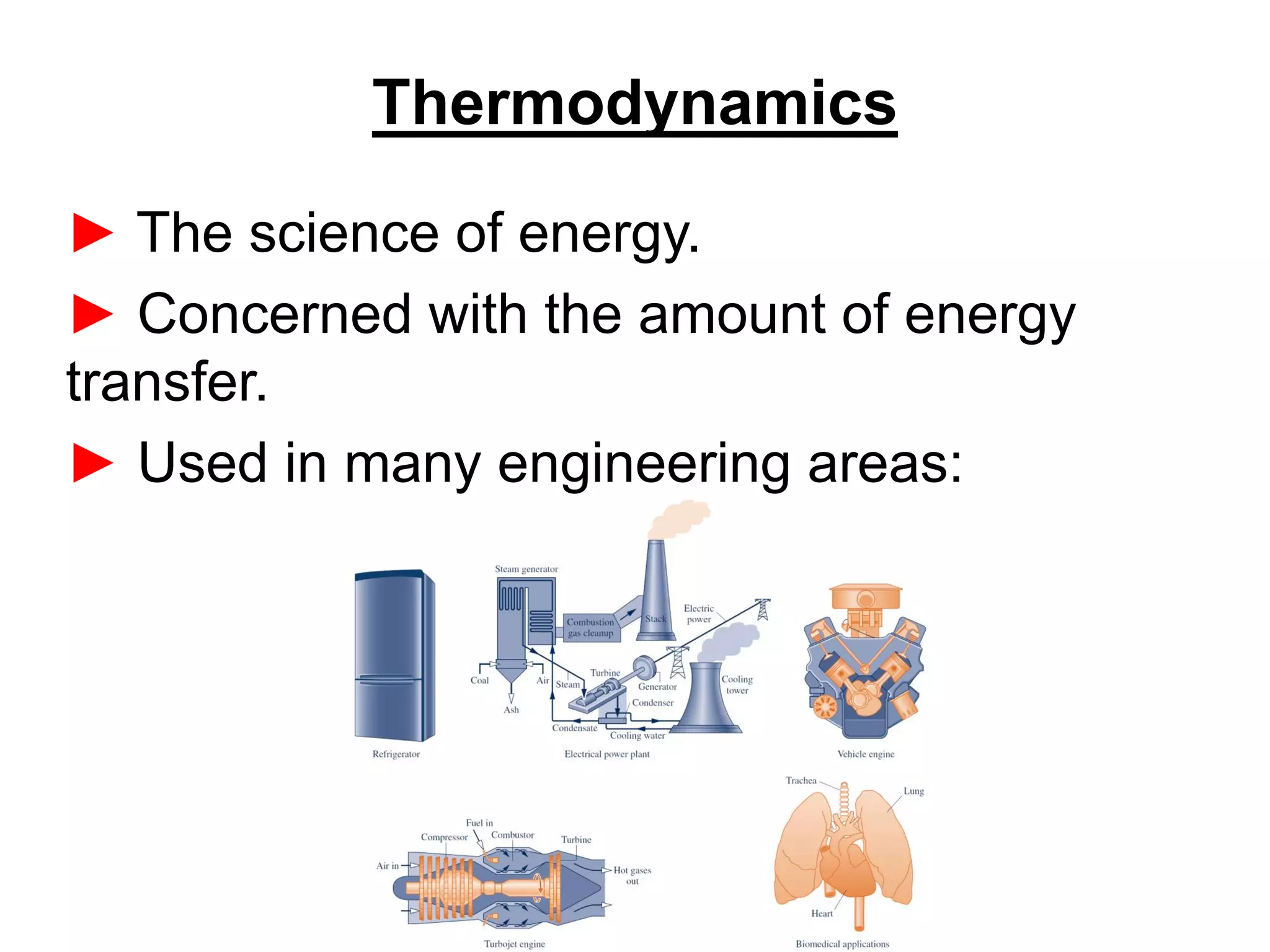
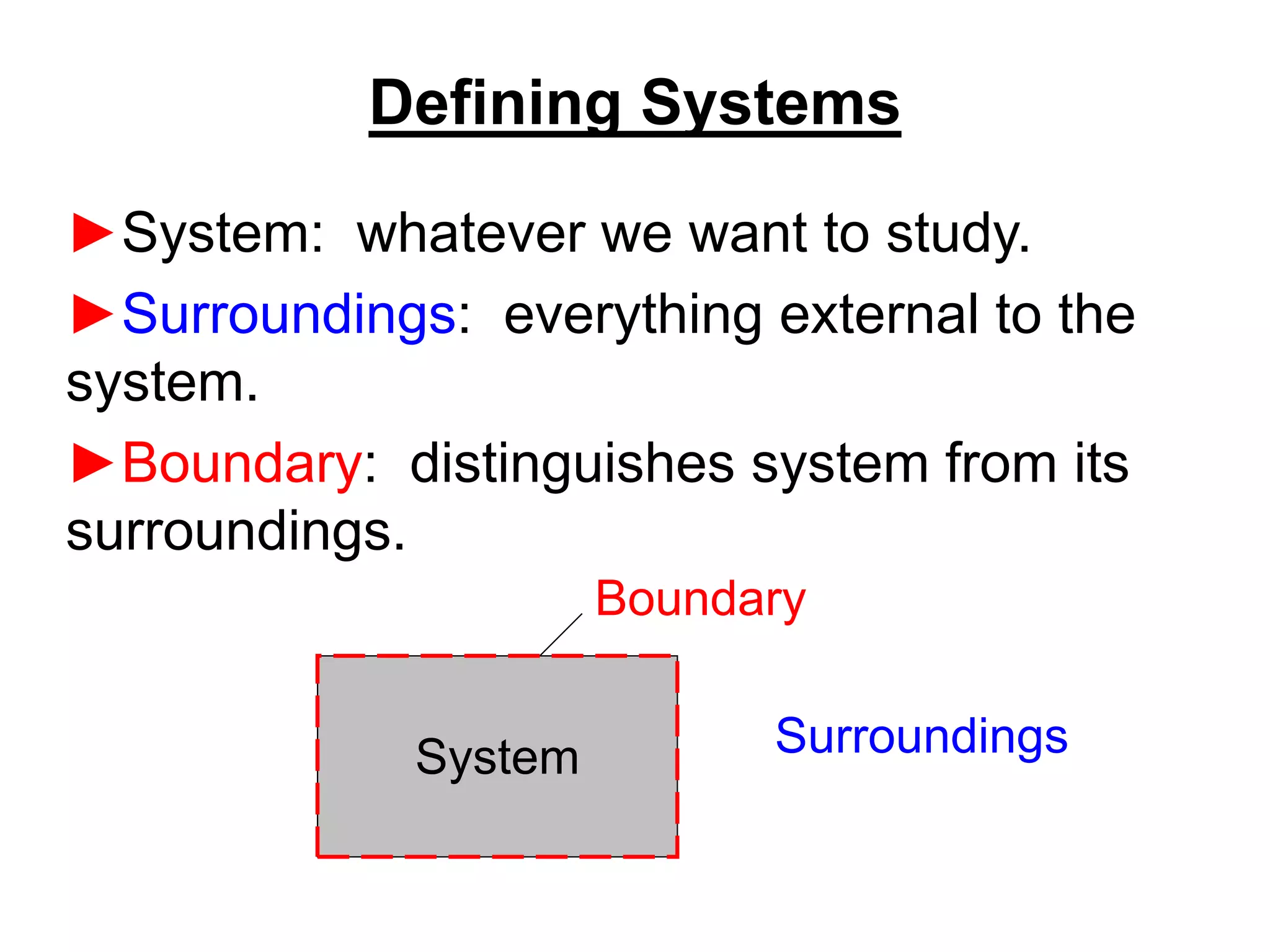
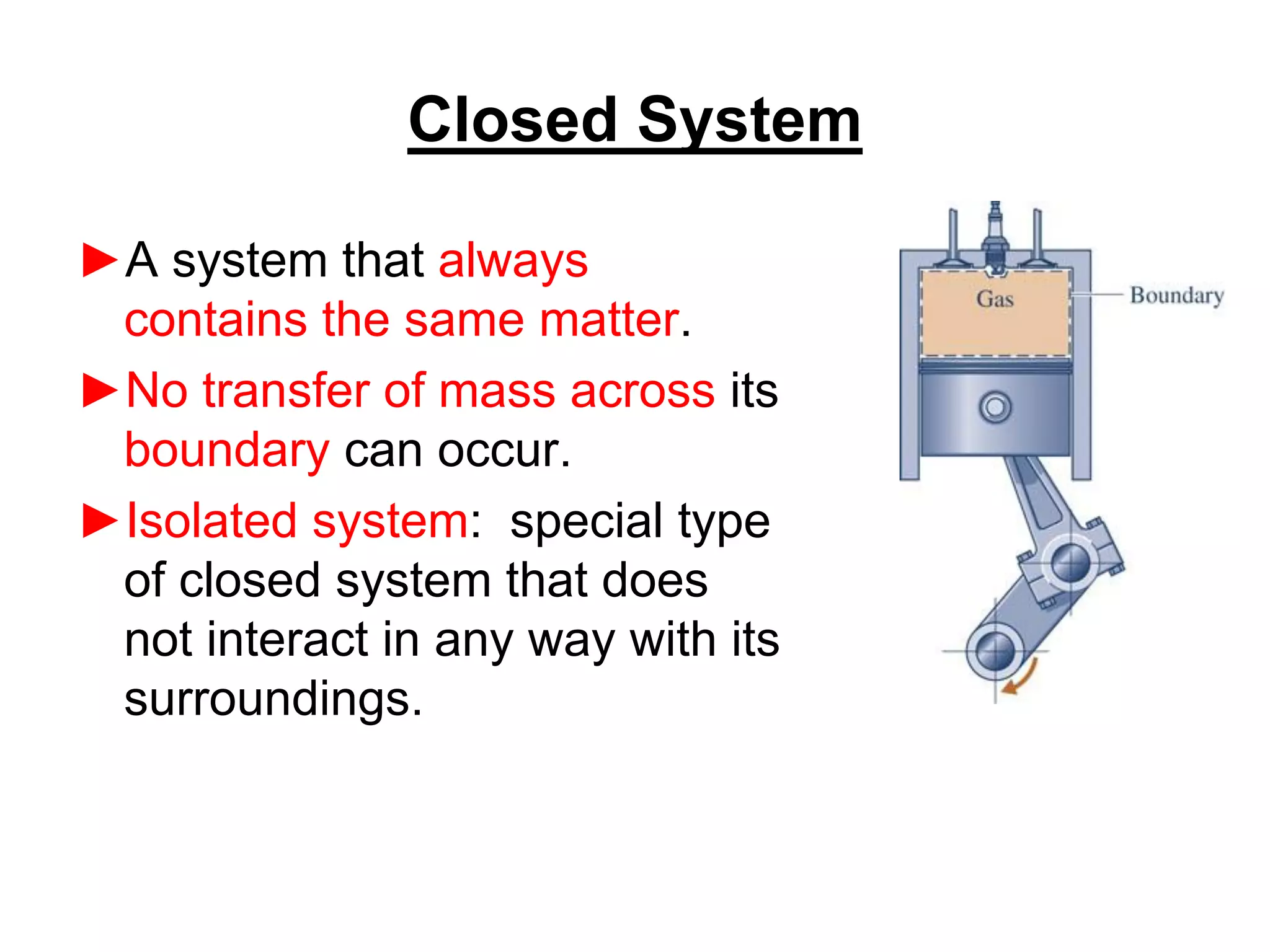
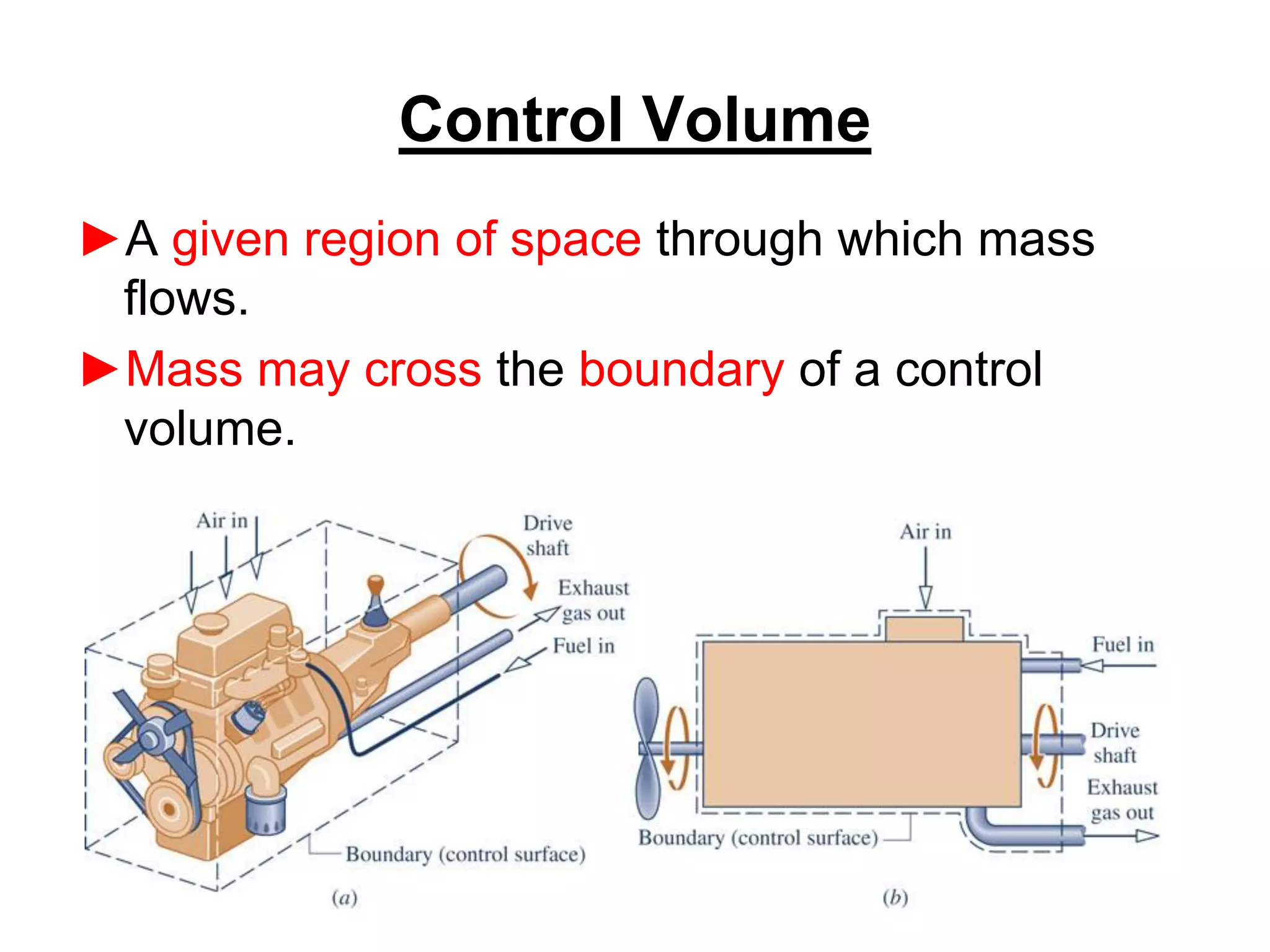
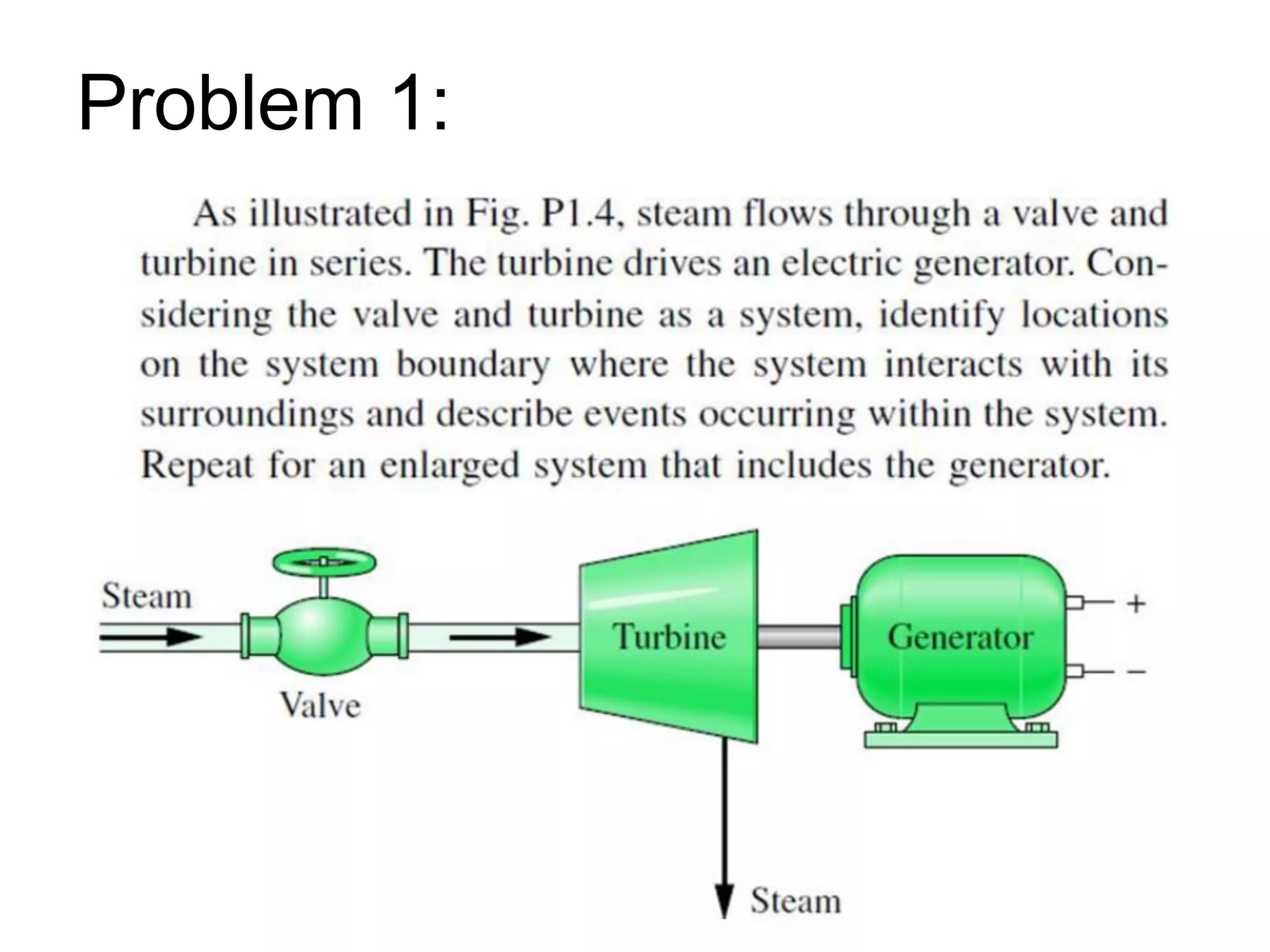
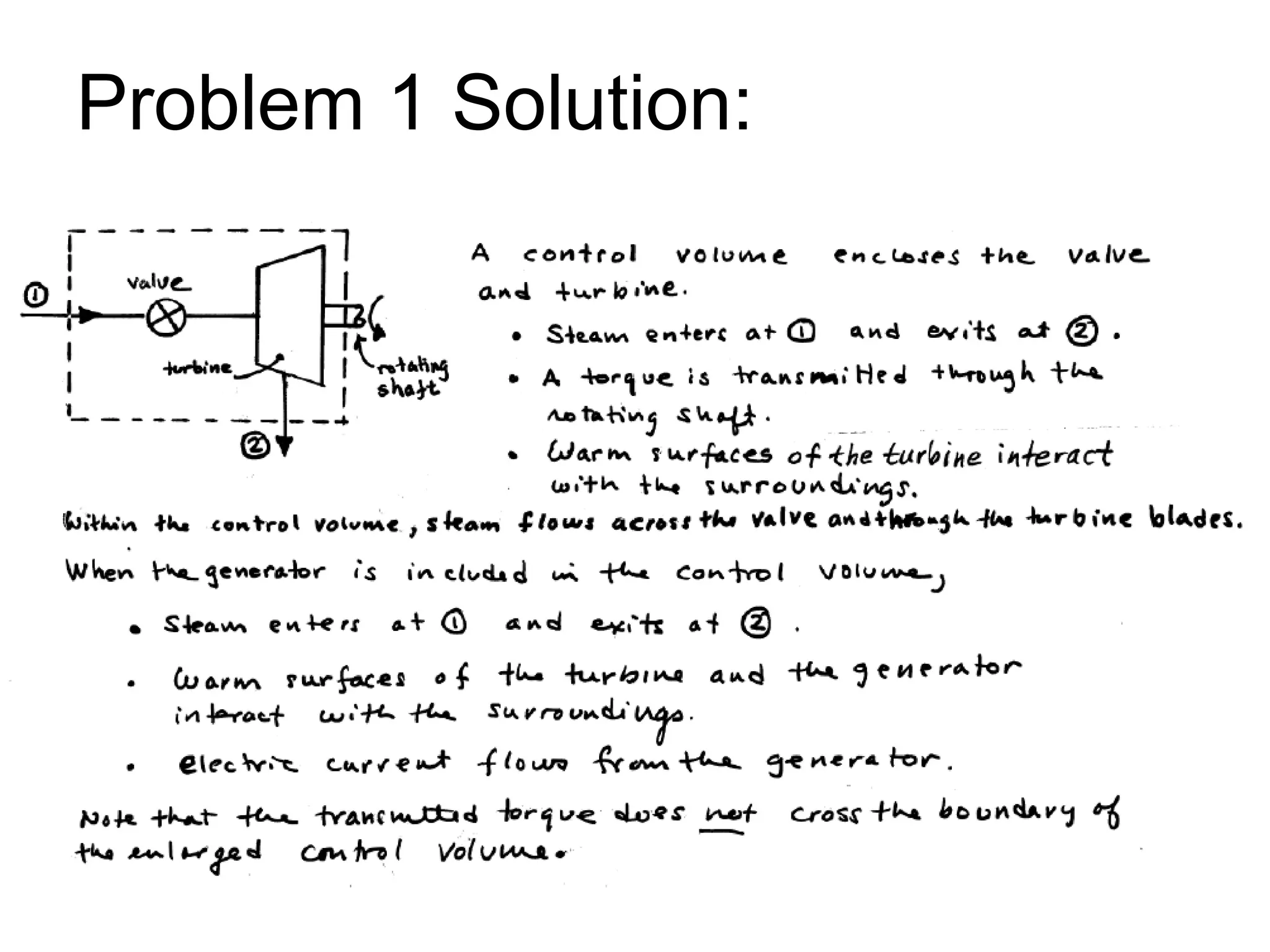
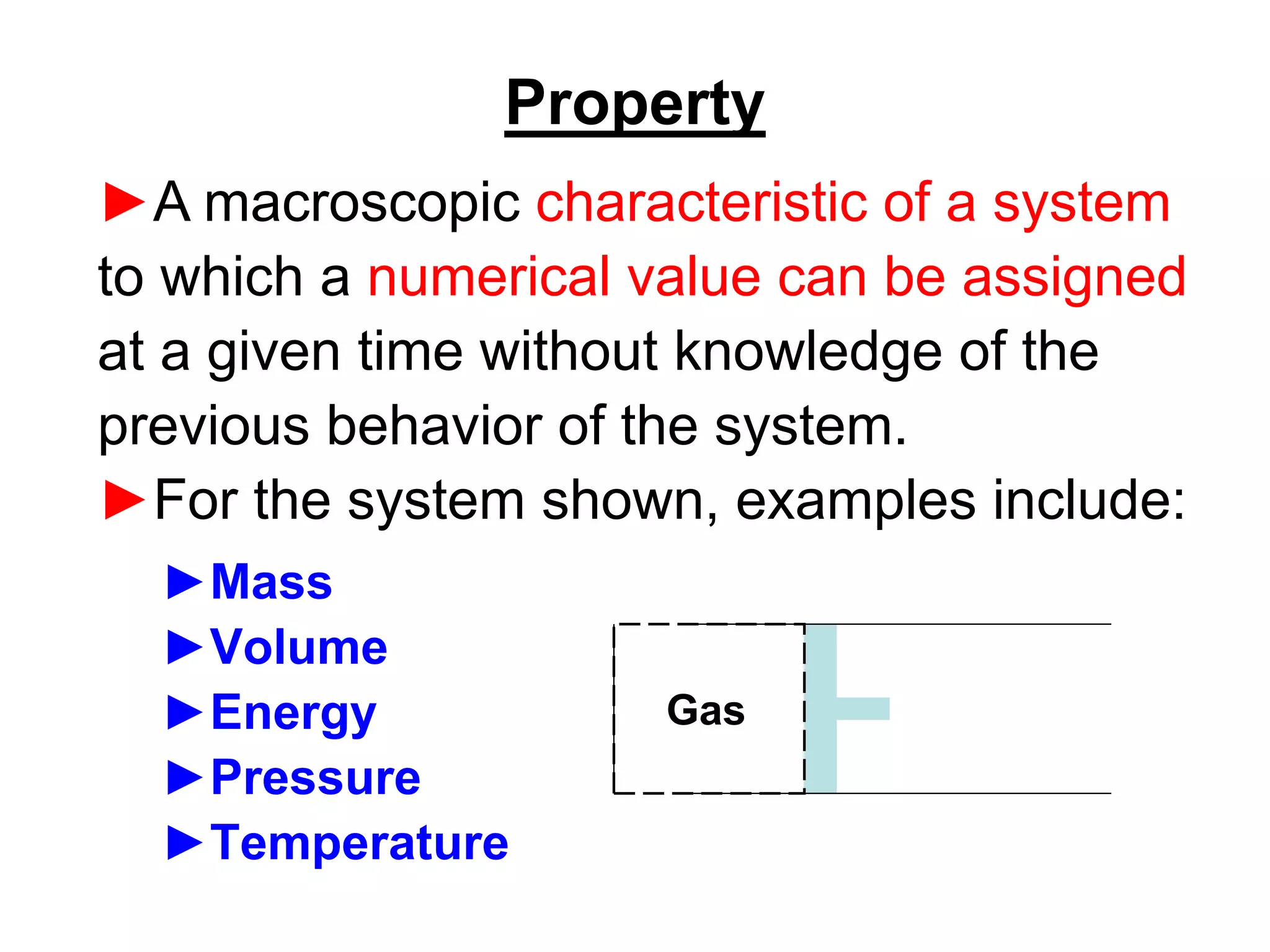
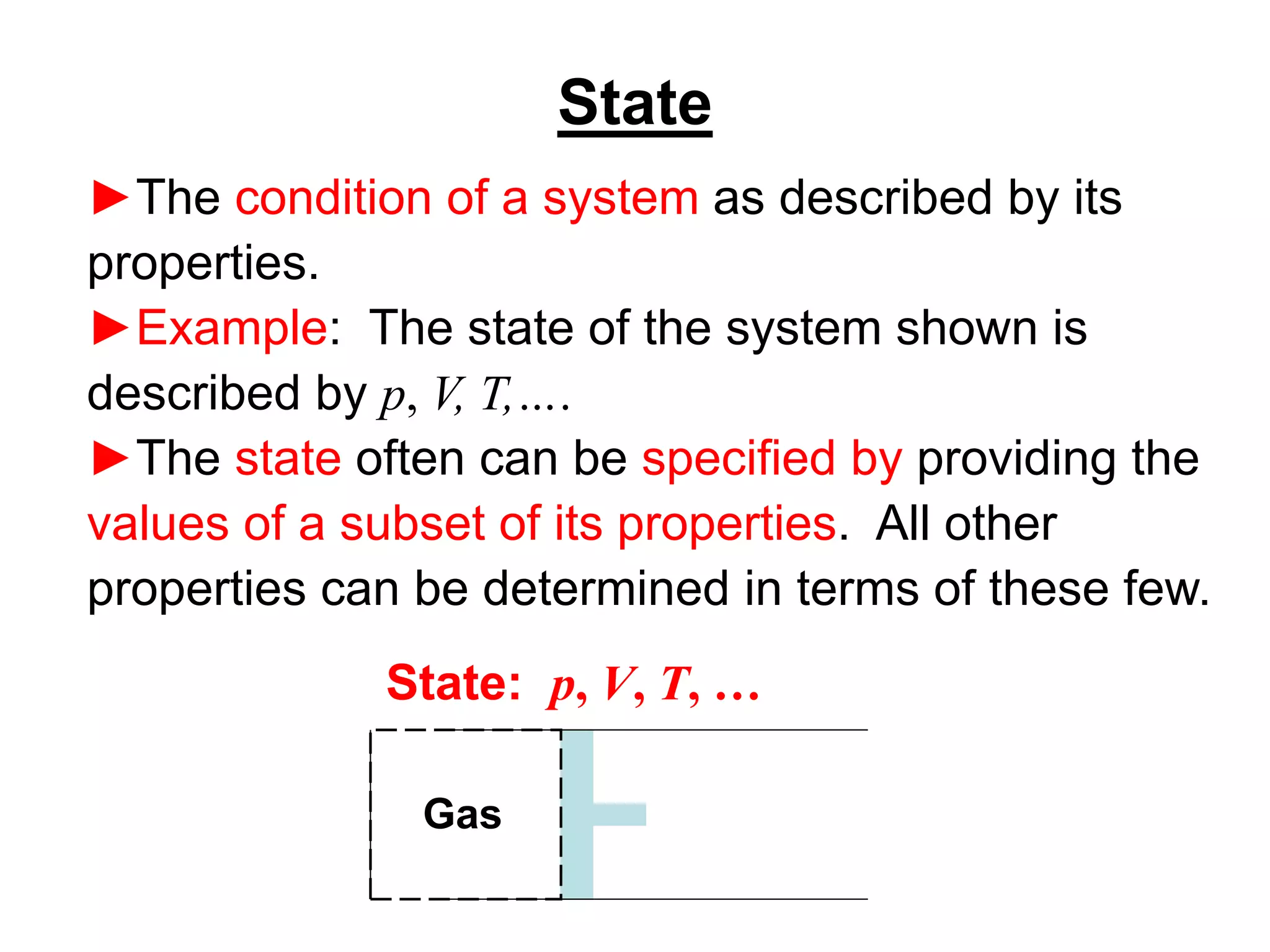
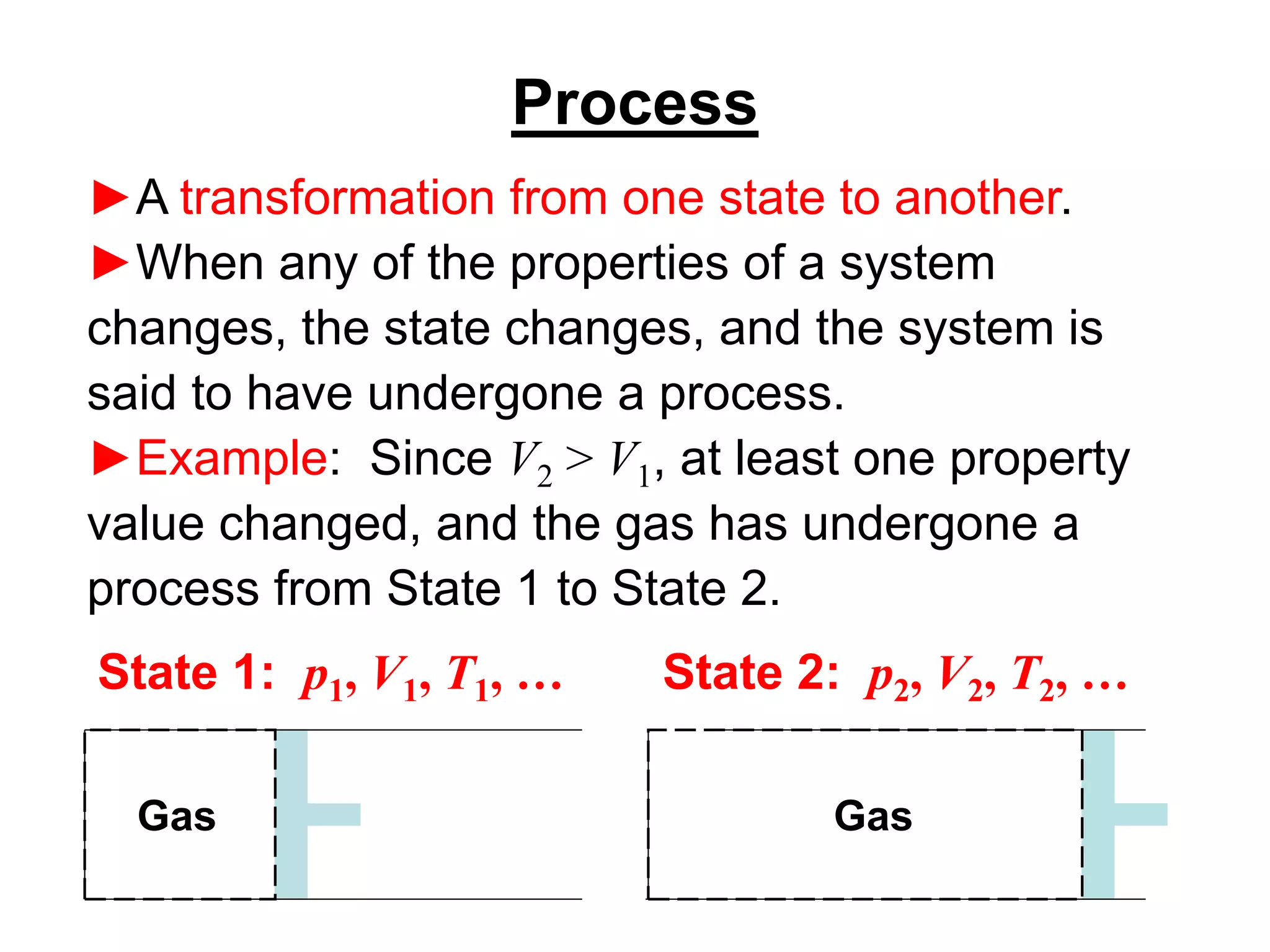
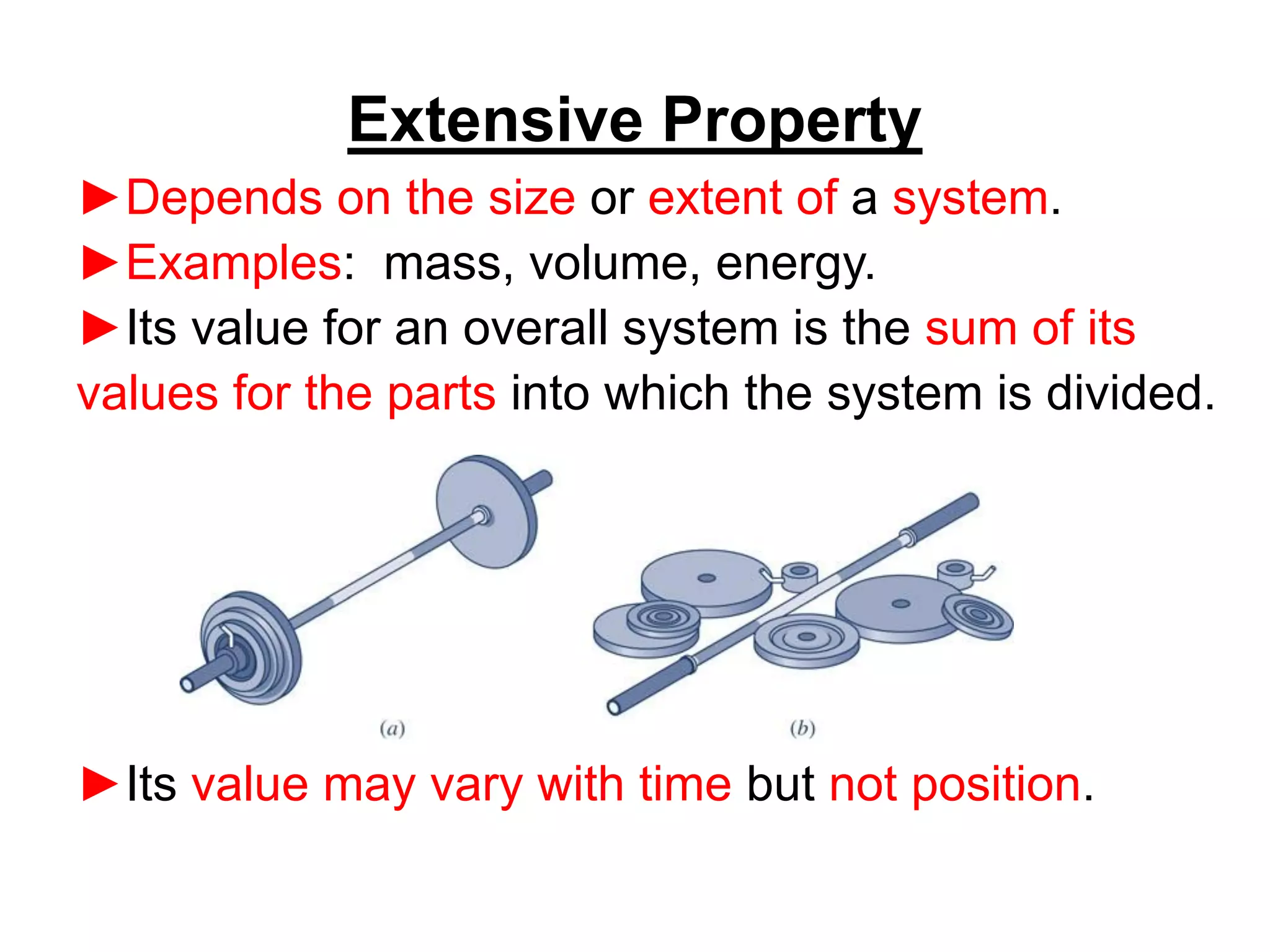
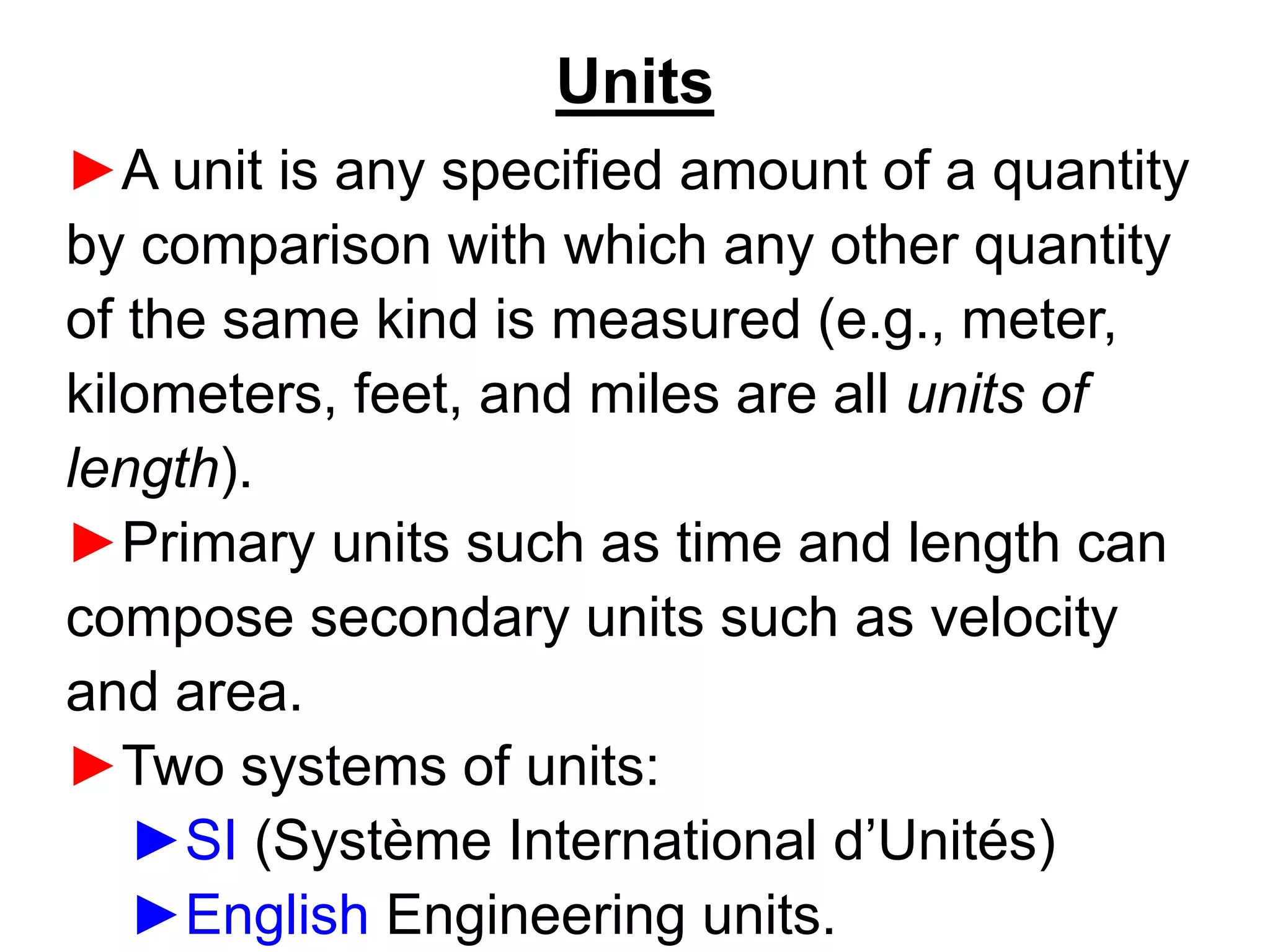
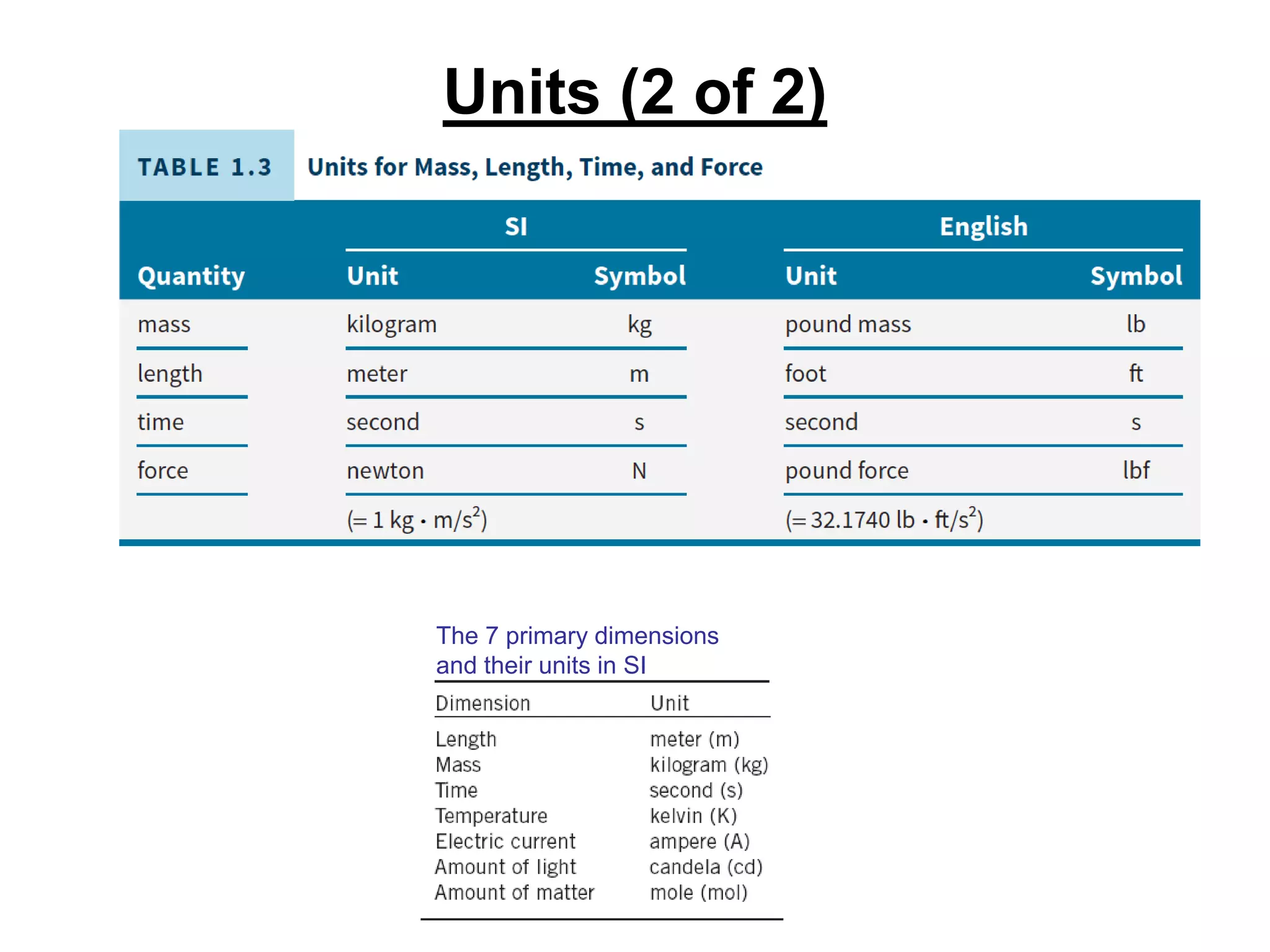
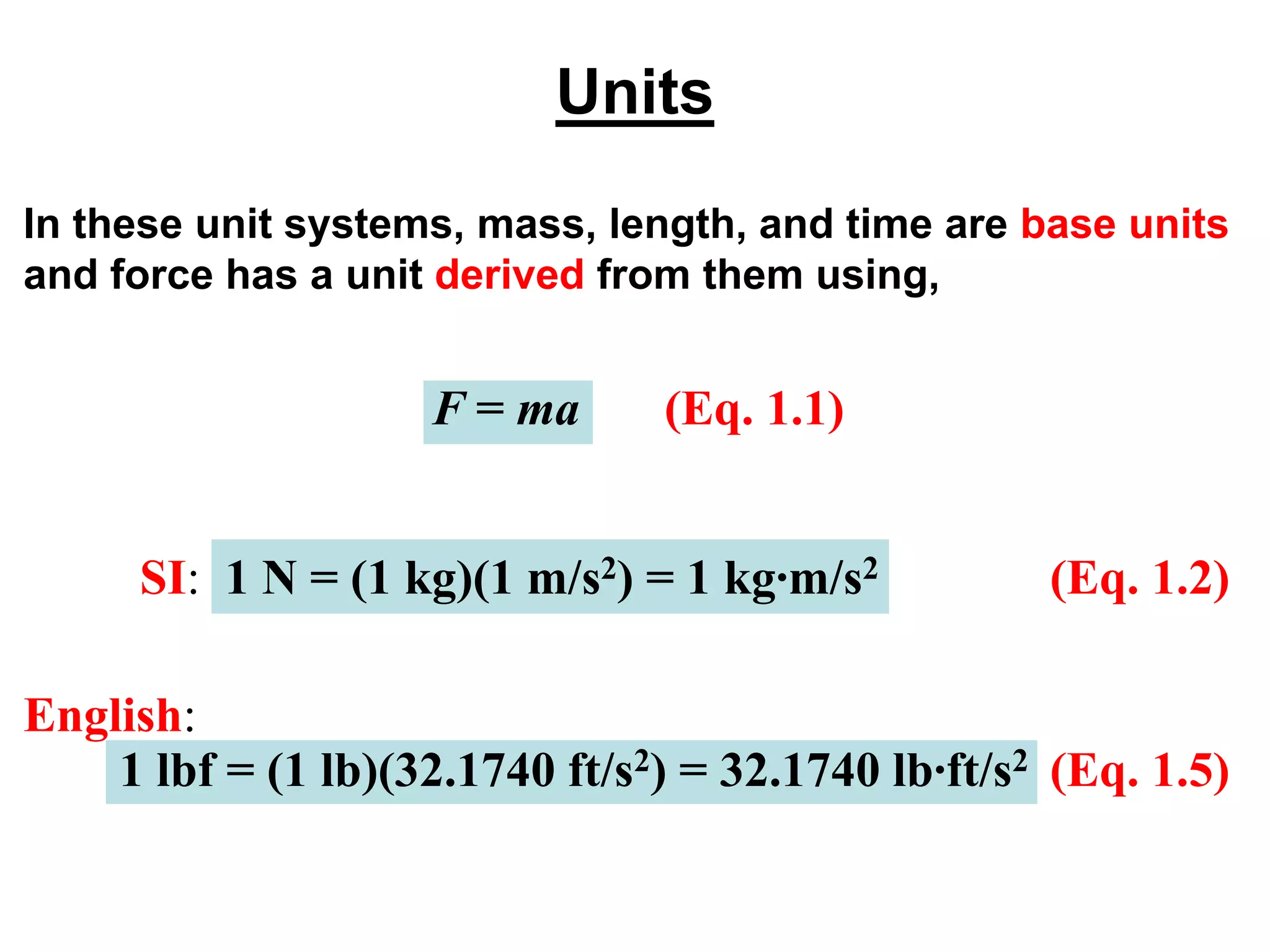
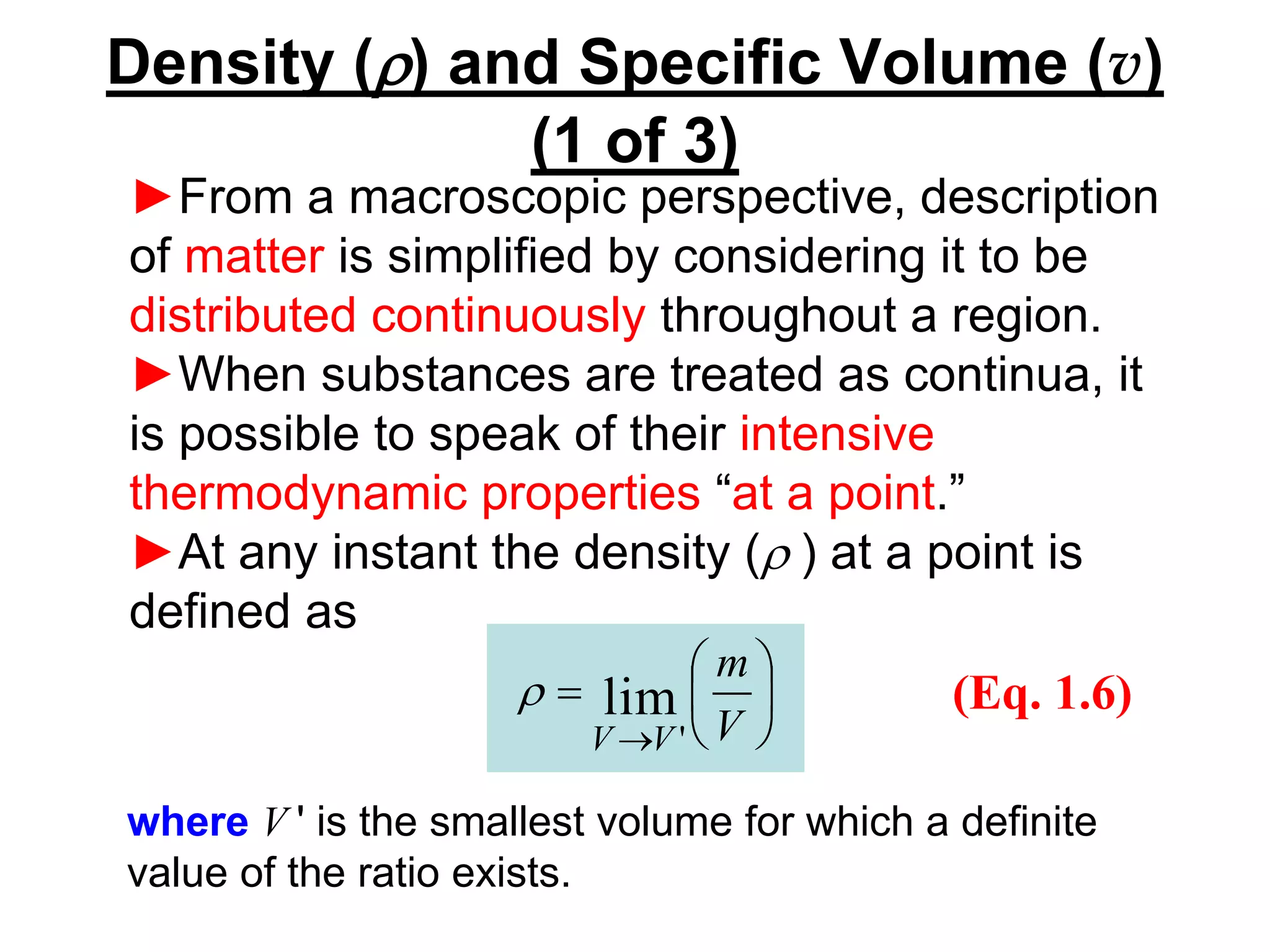
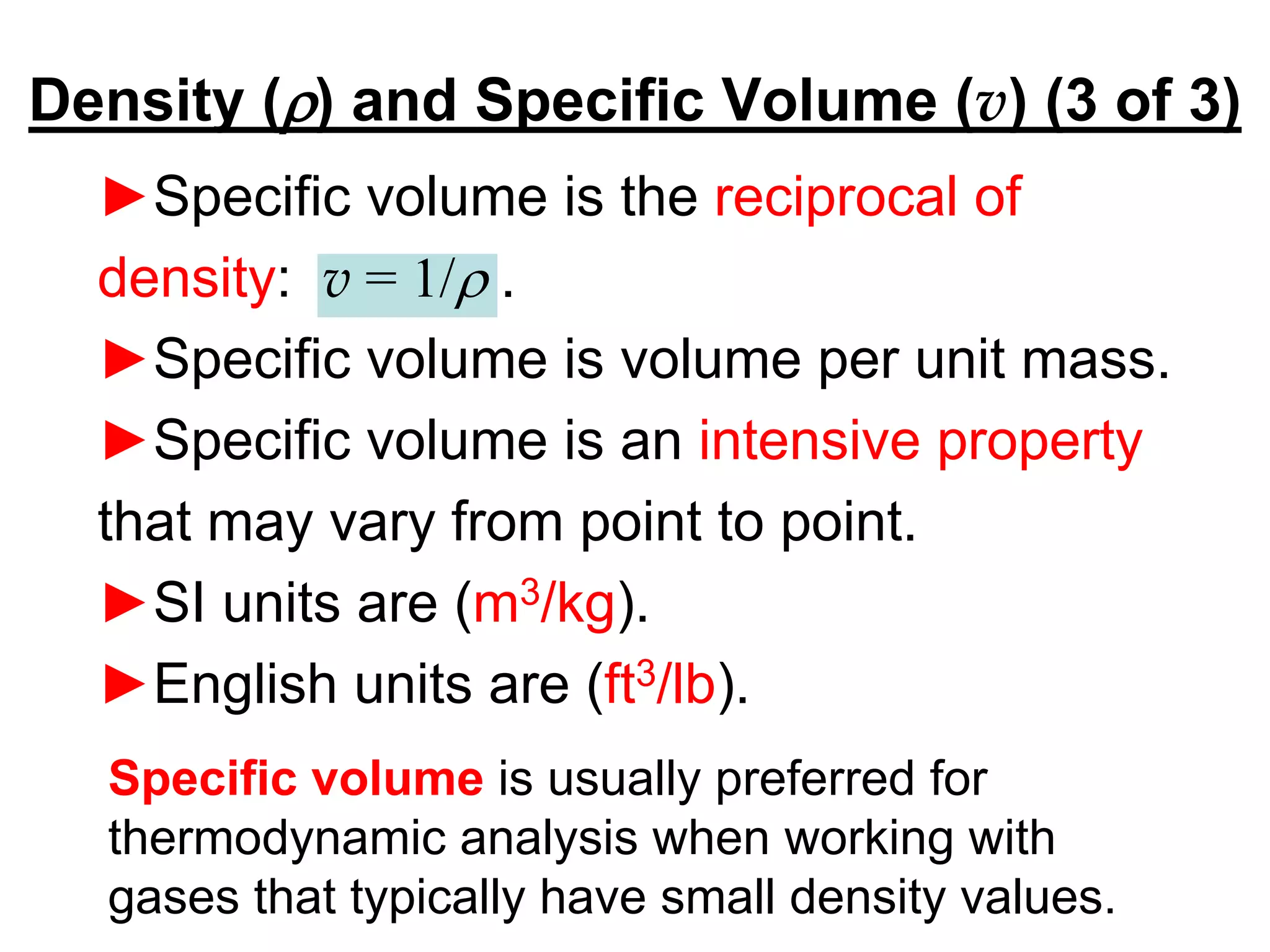
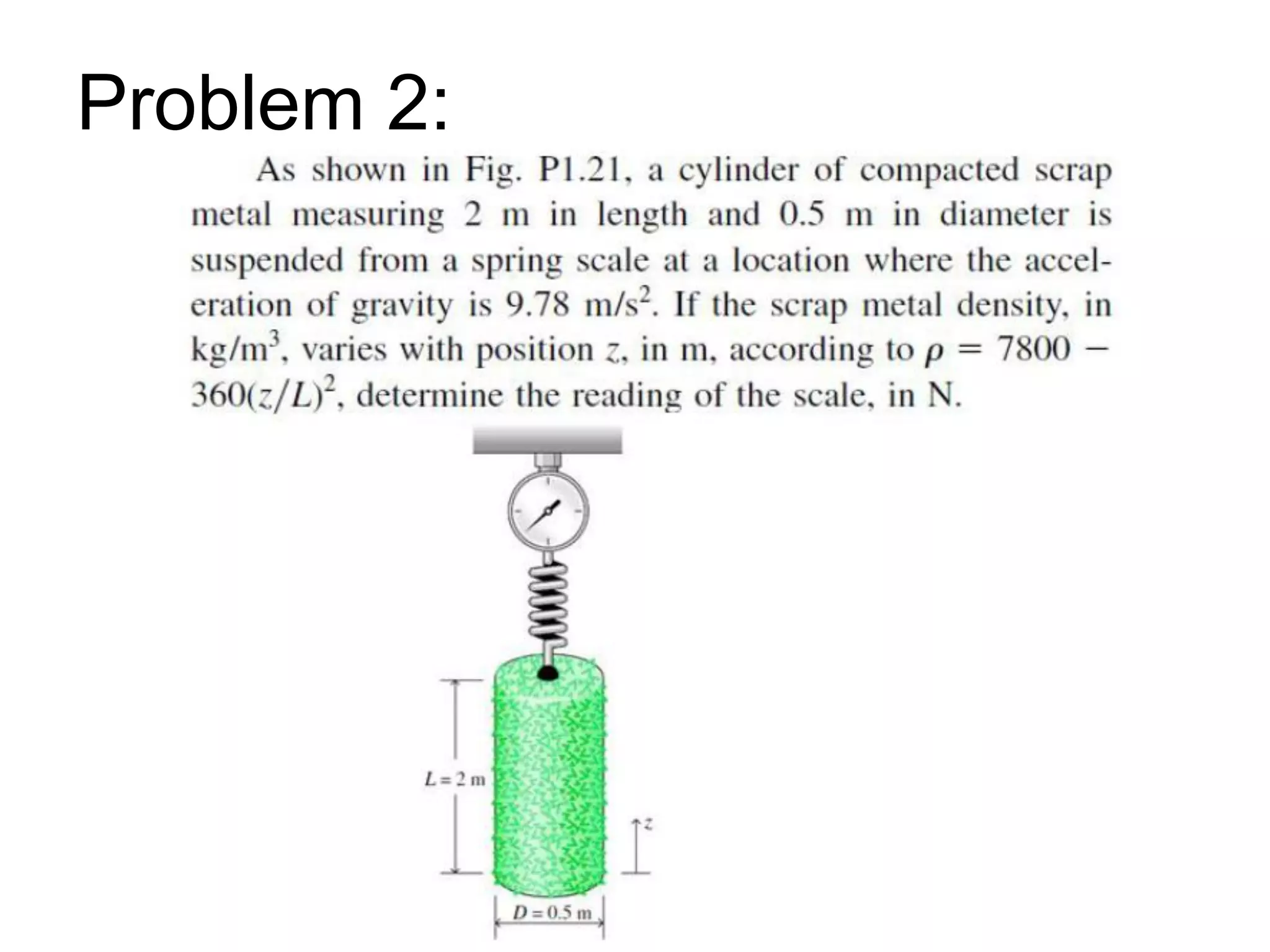
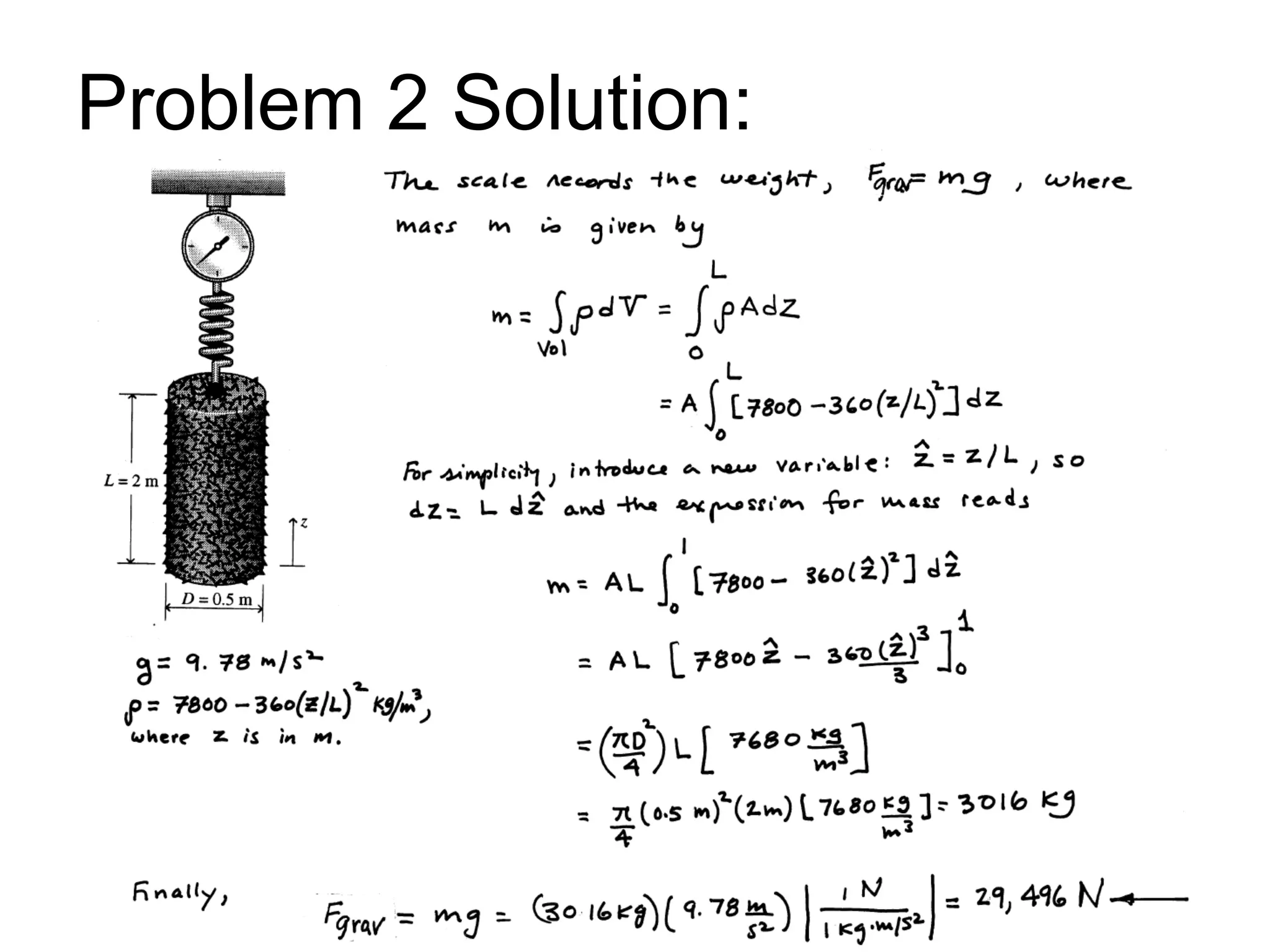
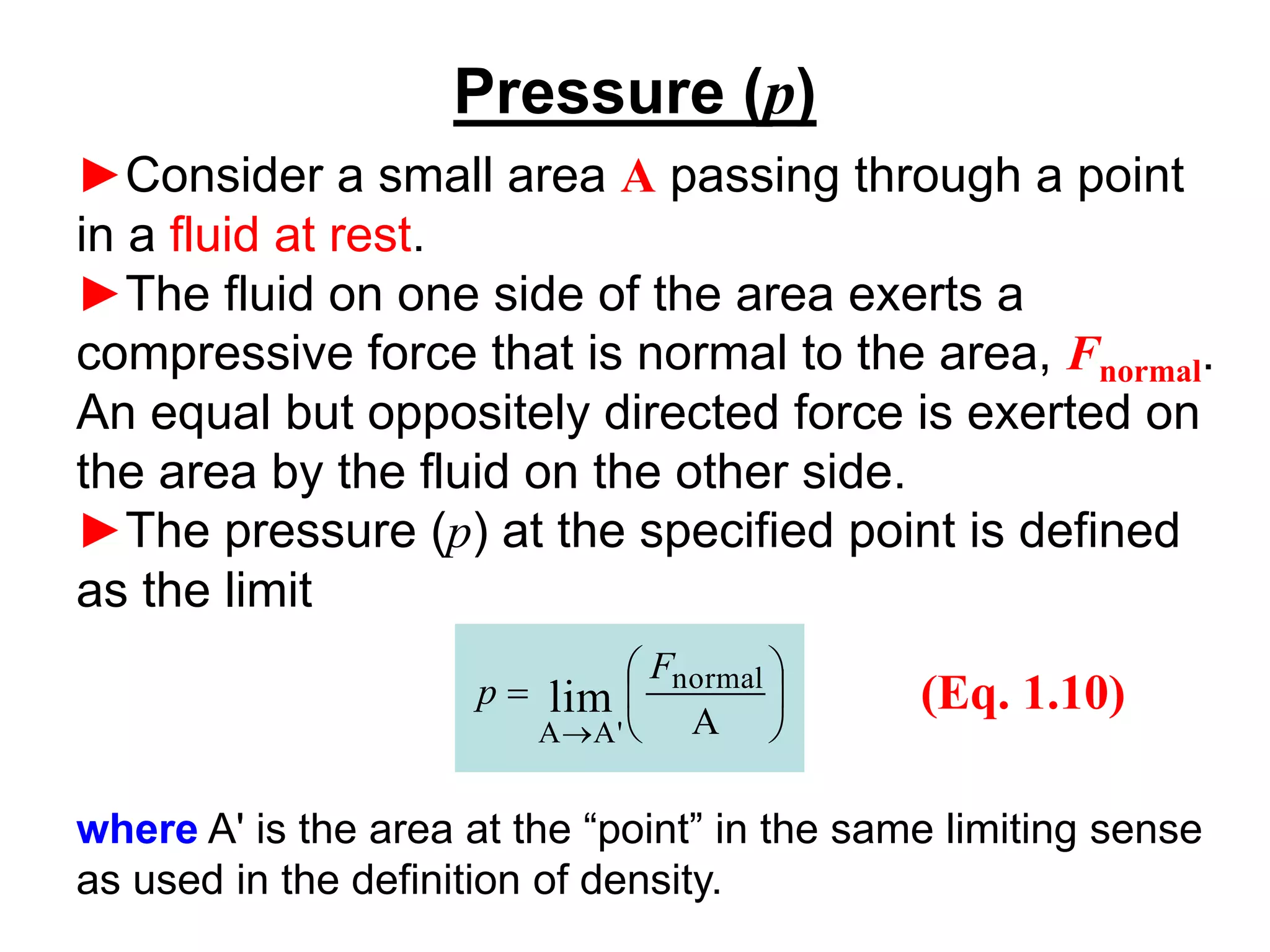
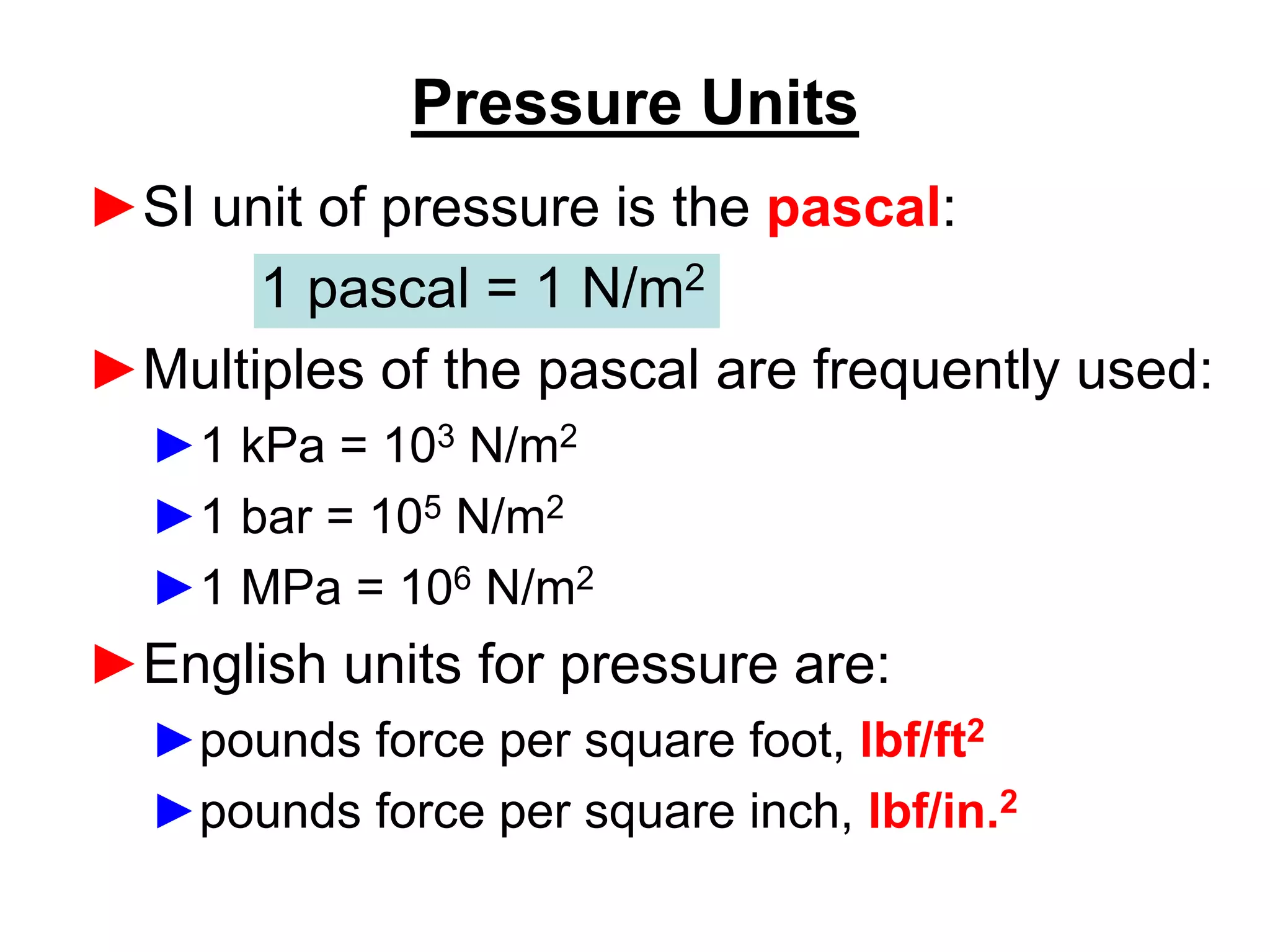
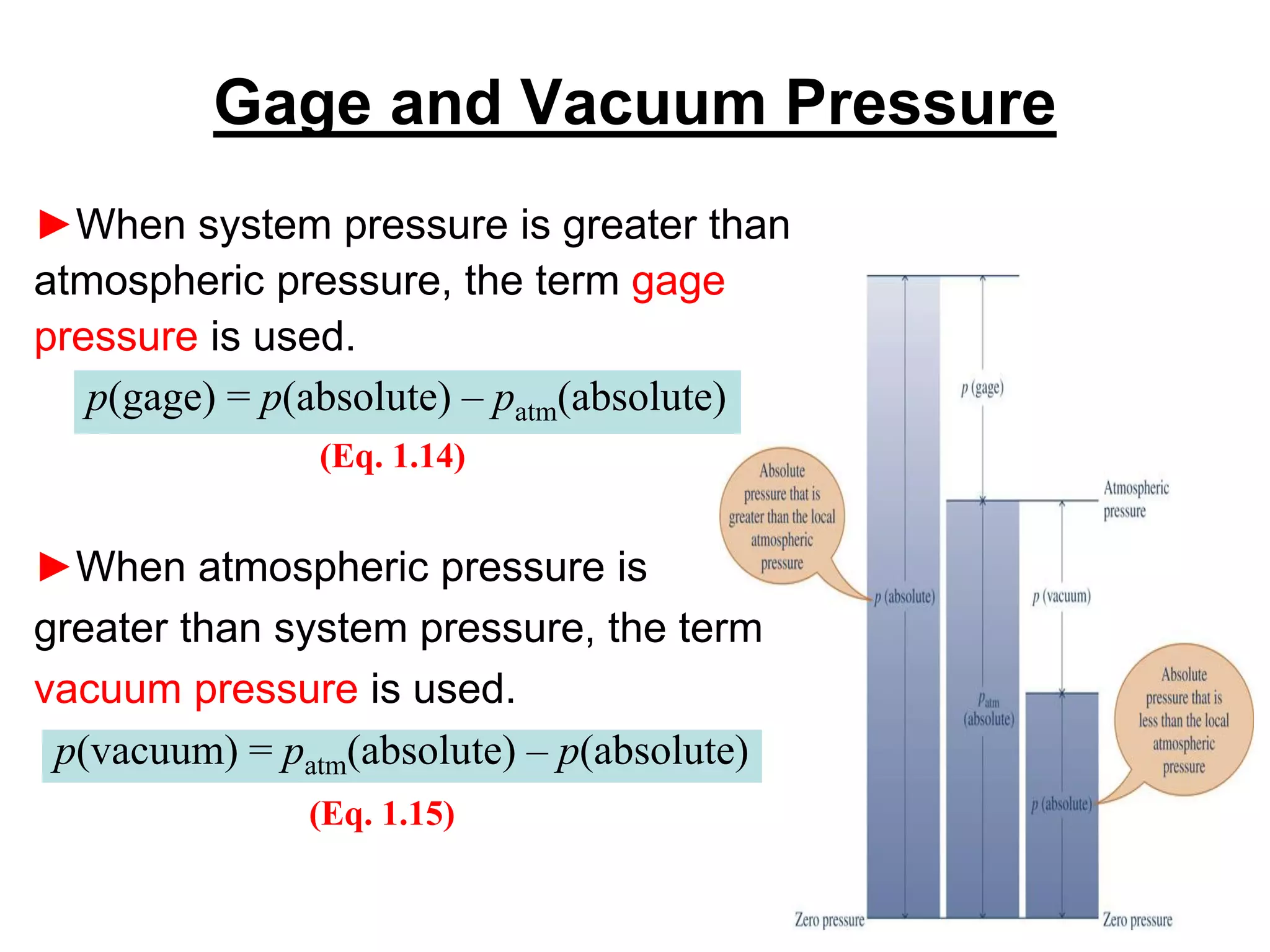
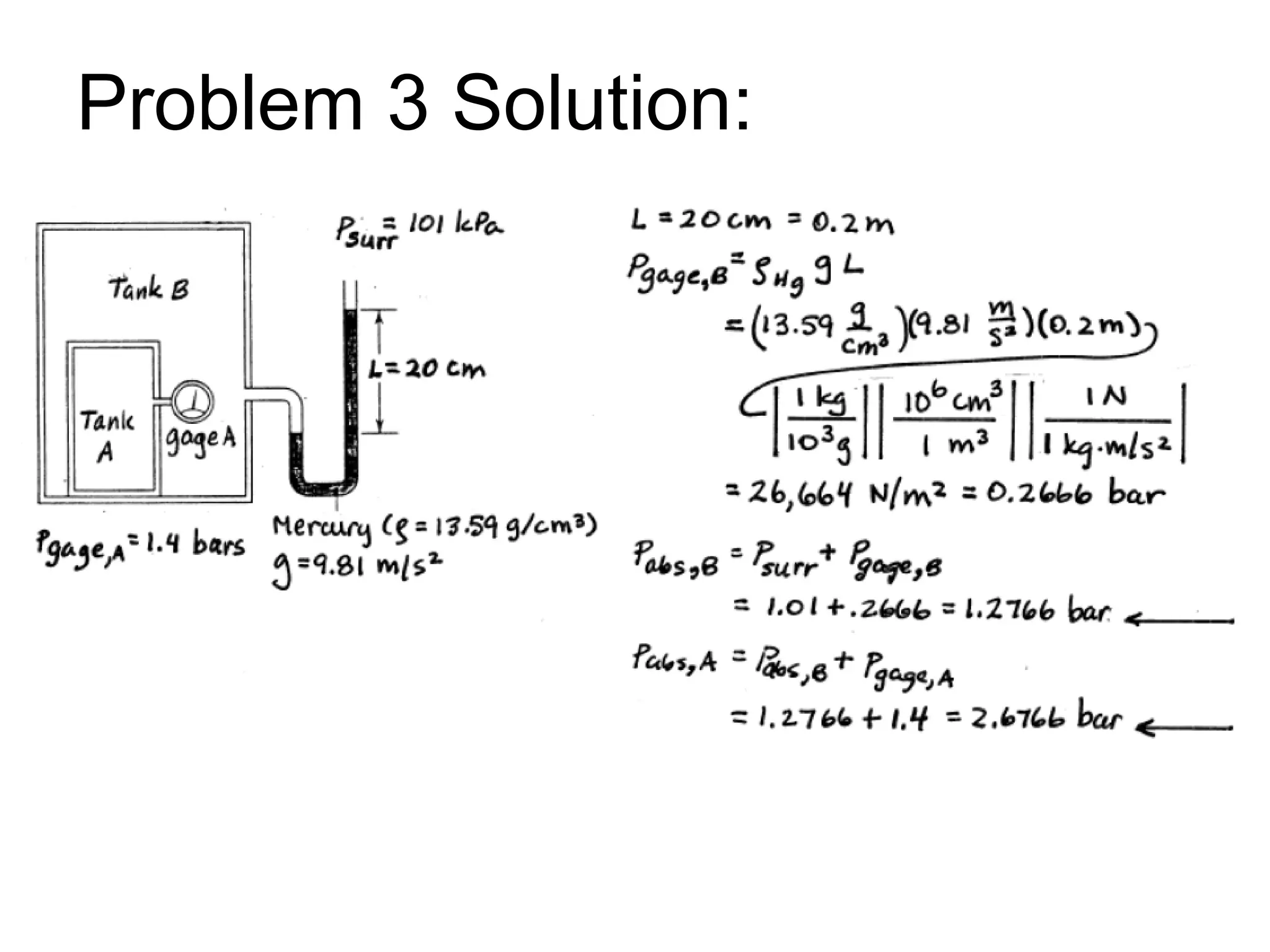
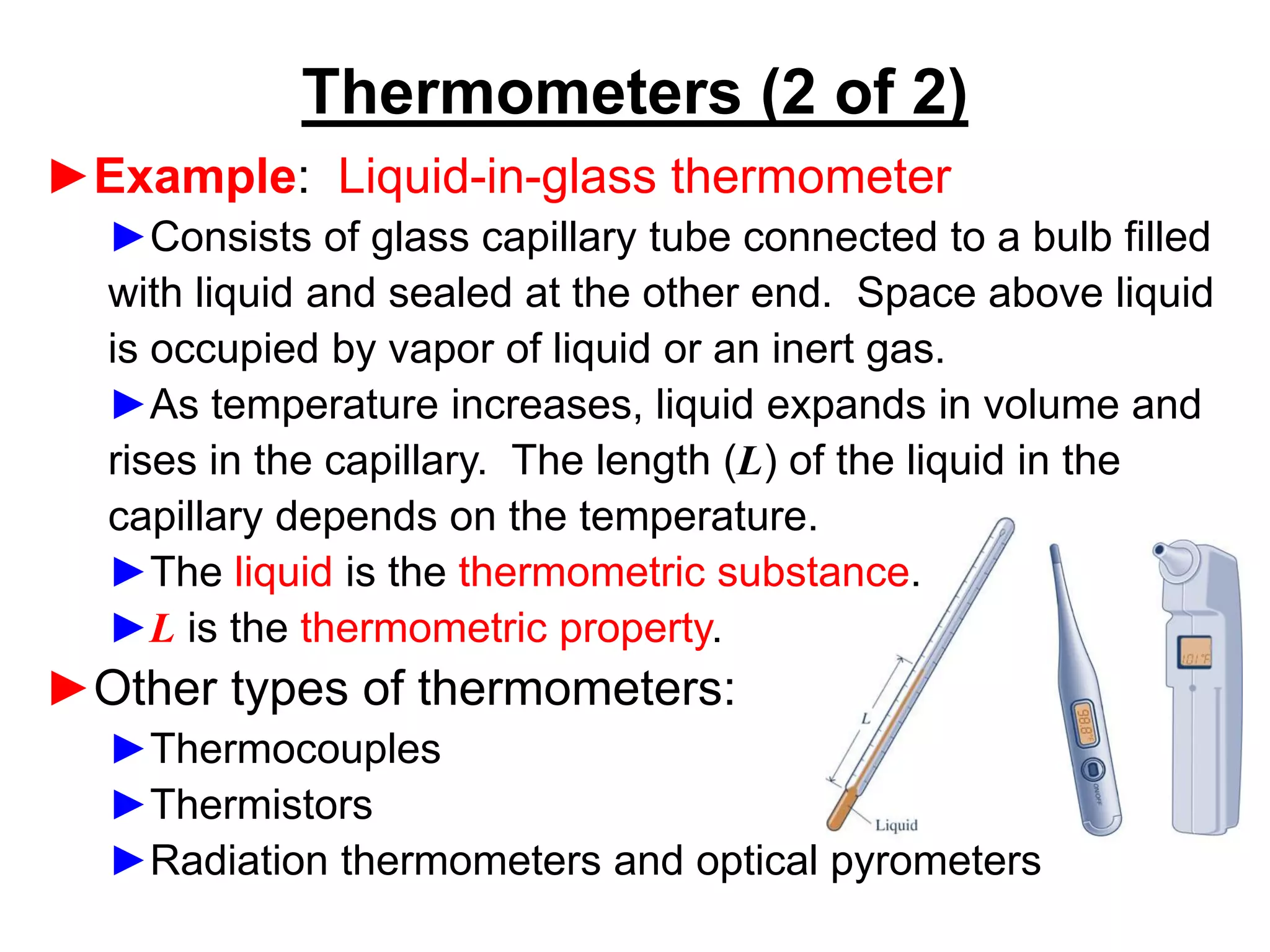
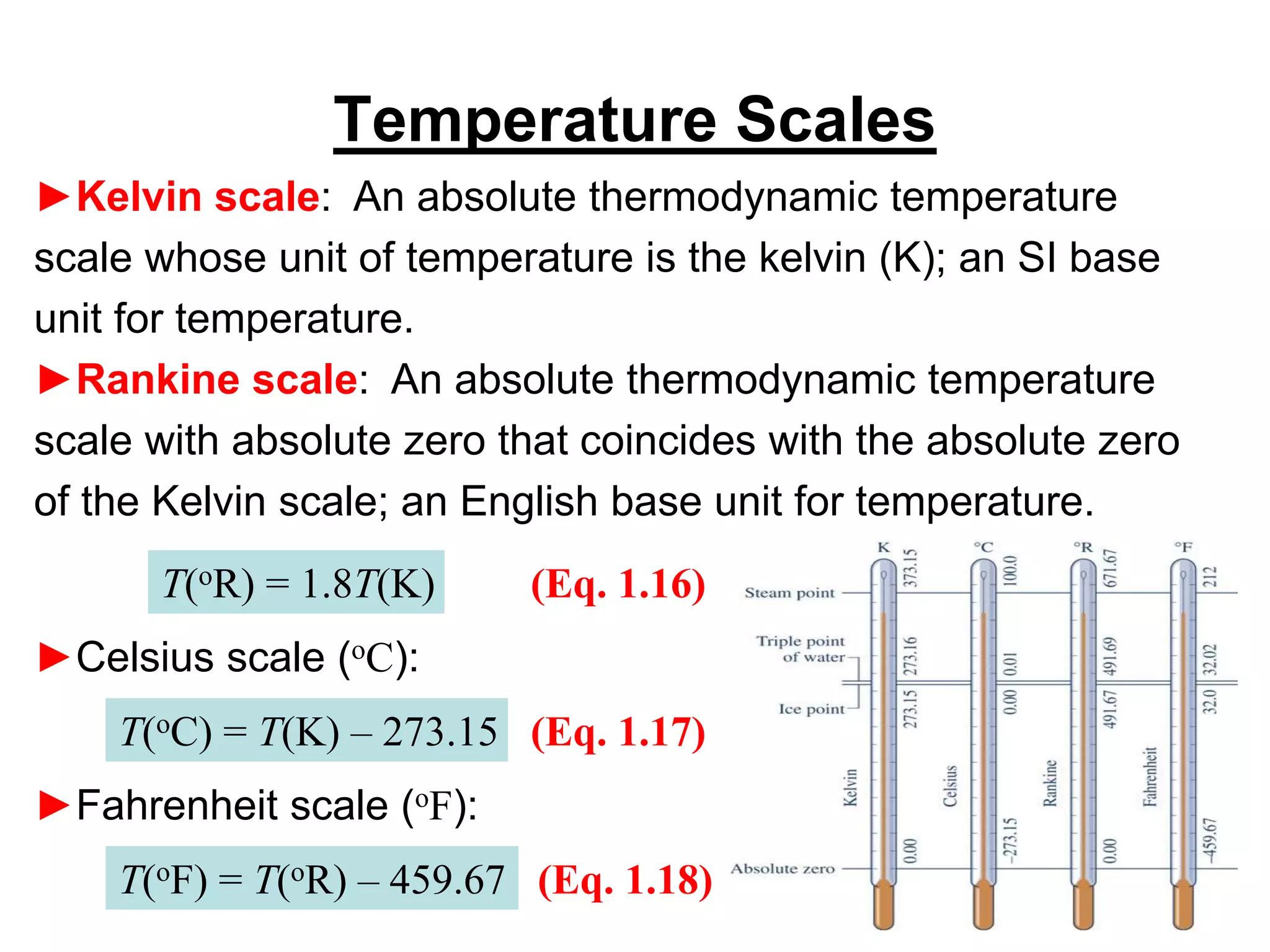
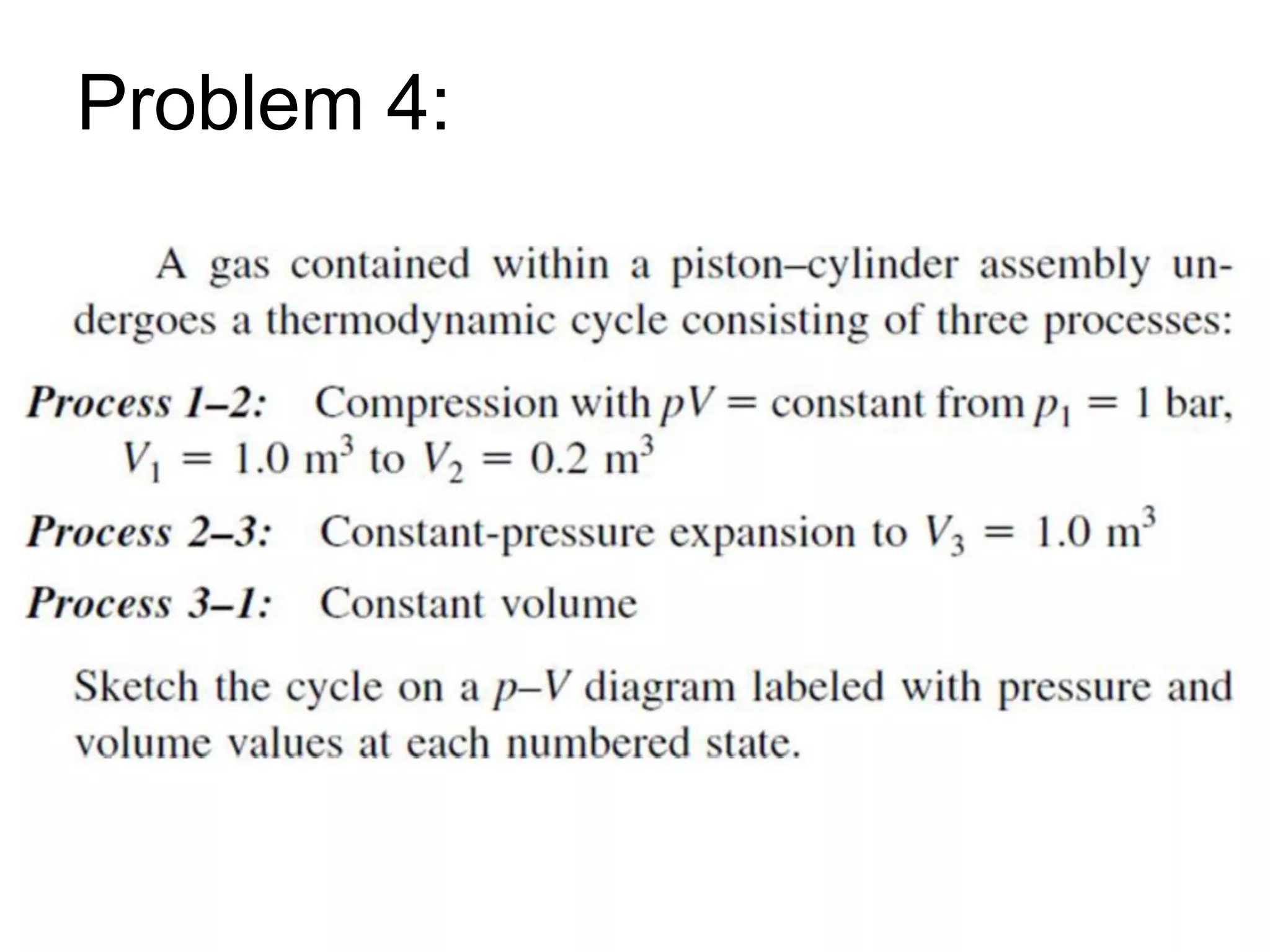
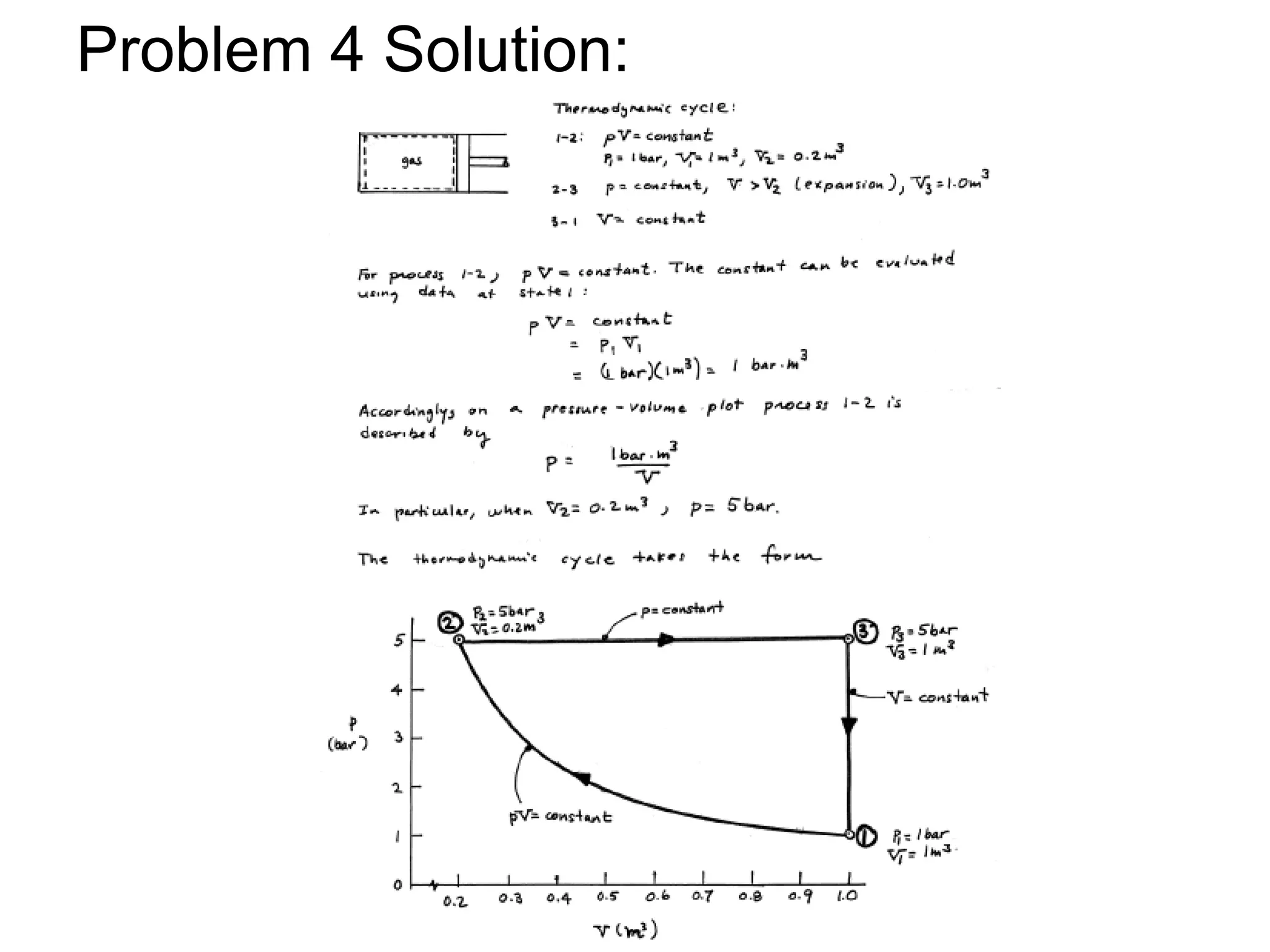
This document provides an outline and overview of key concepts in thermodynamics from chapter 1 of the textbook. It defines fundamental thermodynamic concepts like systems, properties, states, processes, equilibrium, extensive and intensive properties. It also discusses units of measurement for quantities like mass, length, time, force, pressure, temperature and defines related concepts like density, specific volume, absolute and gauge pressure. The chapter aims to explain these introductory concepts needed for thermodynamic analysis.