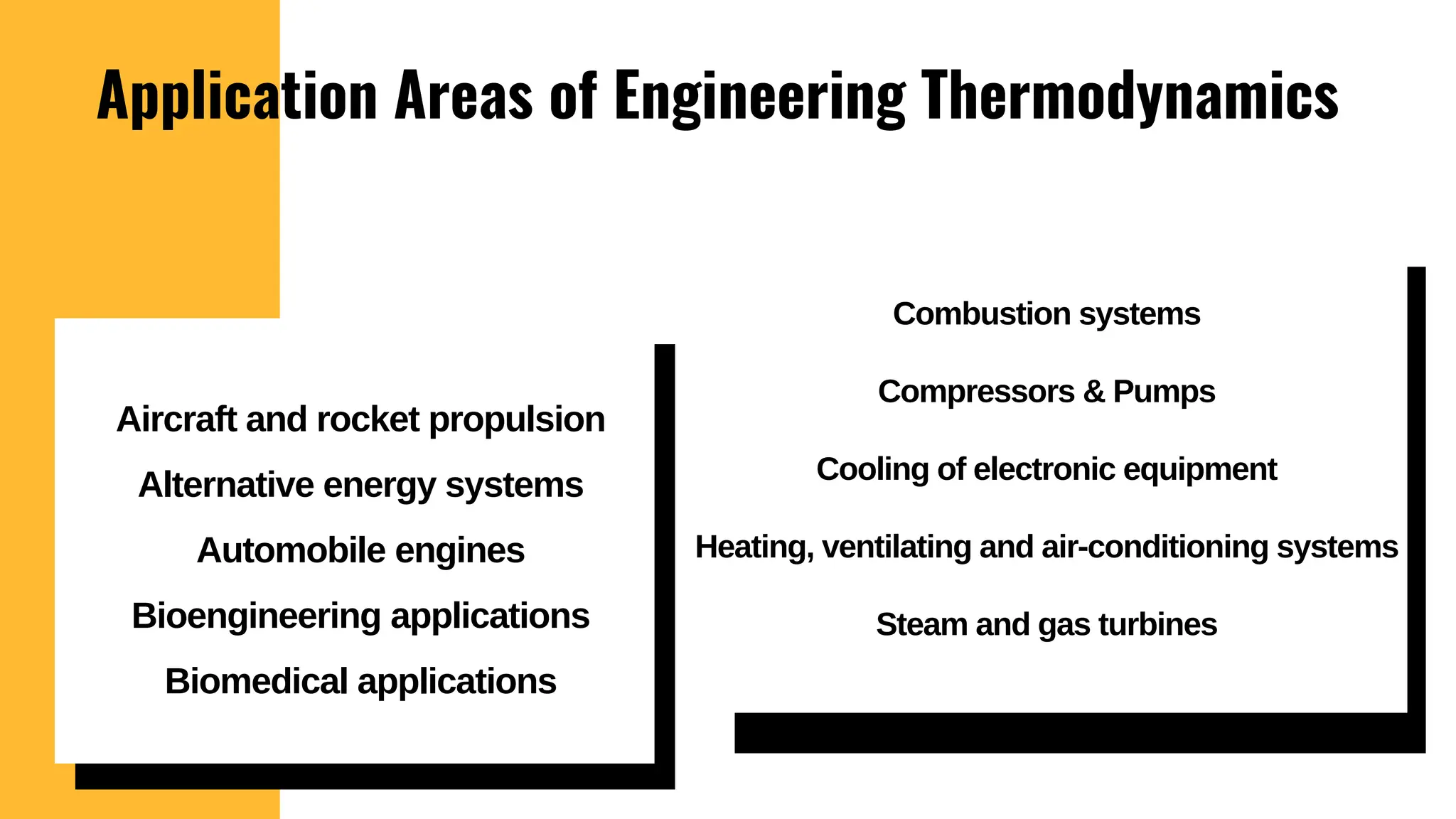
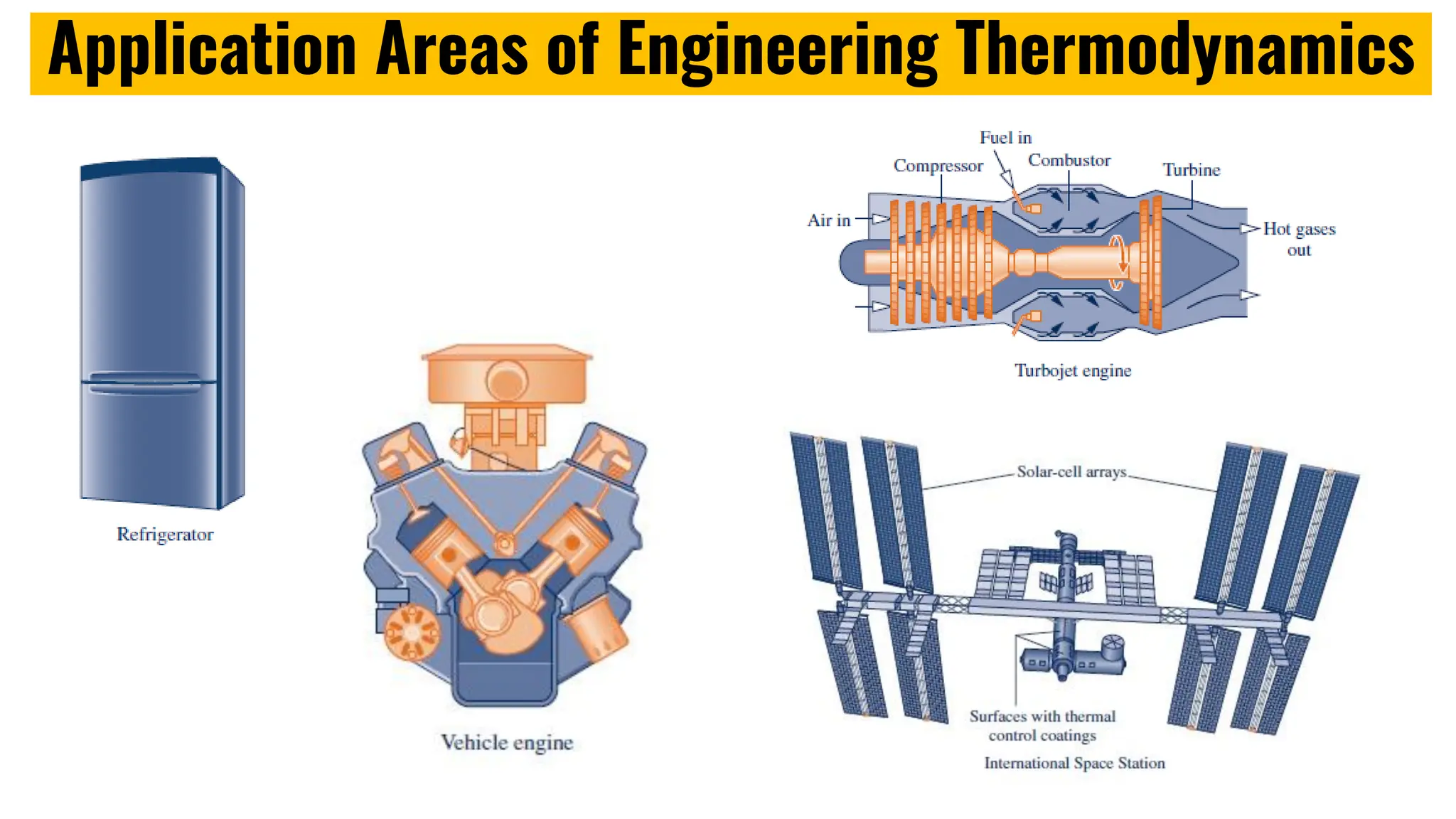
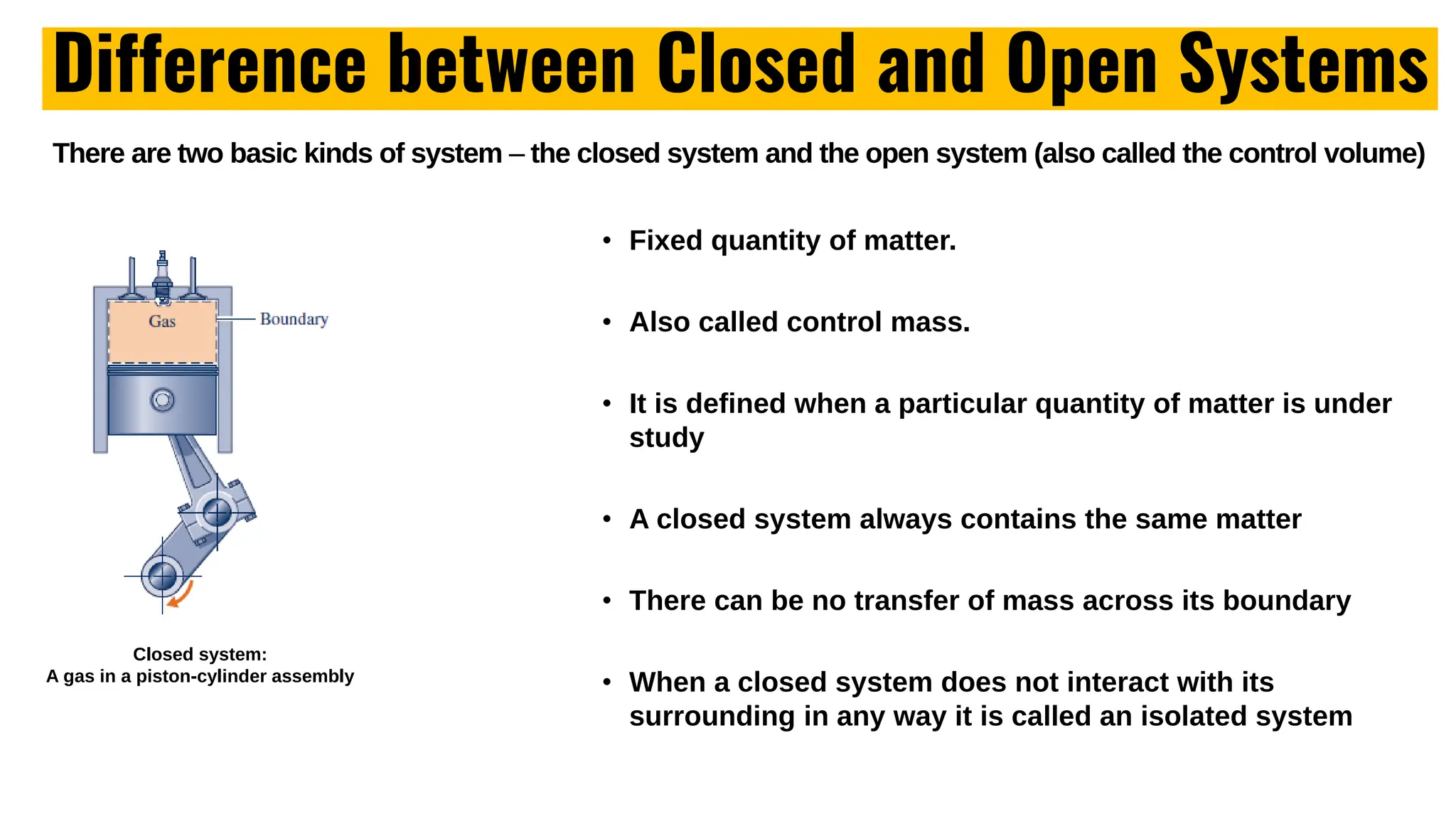
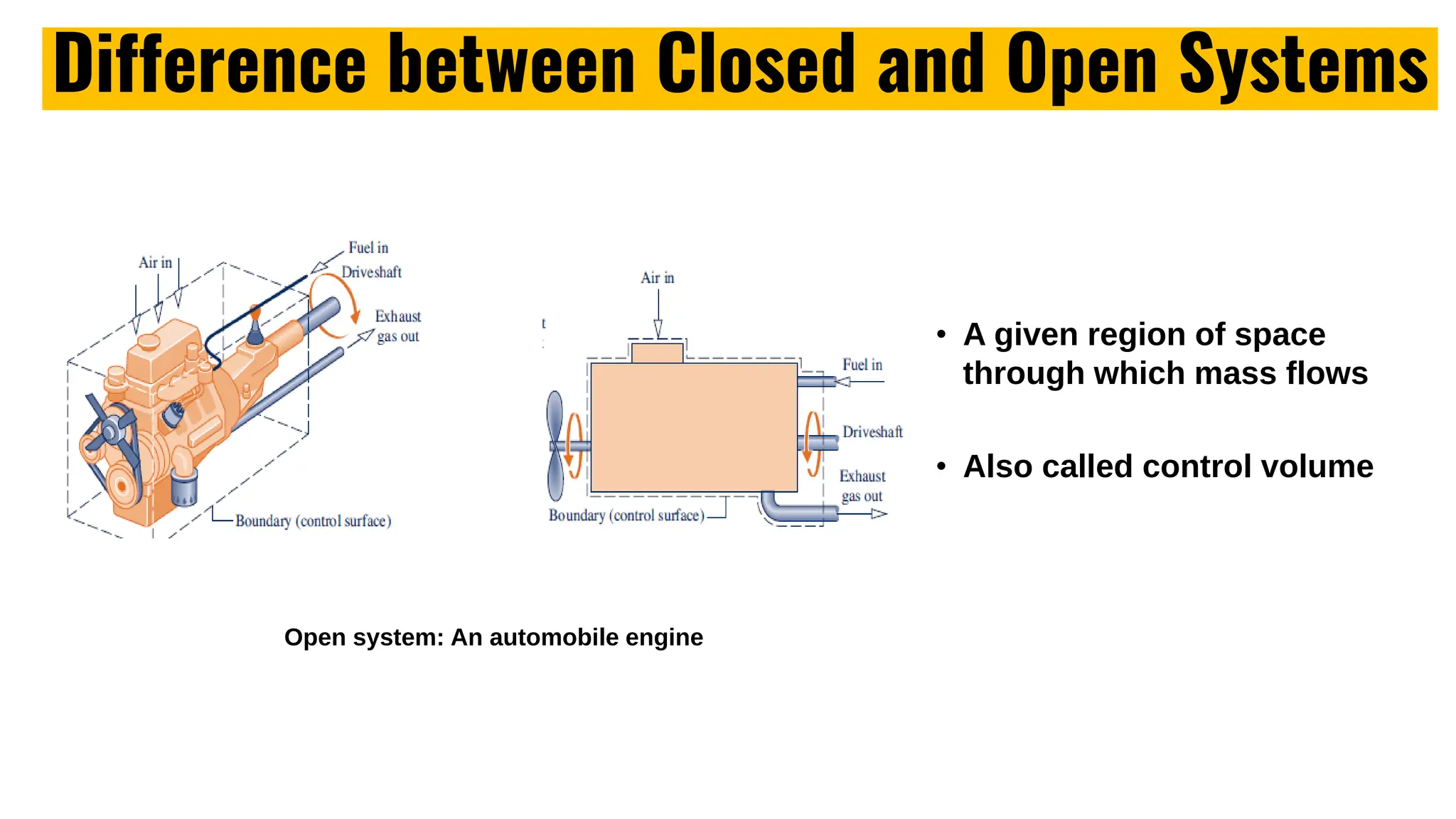
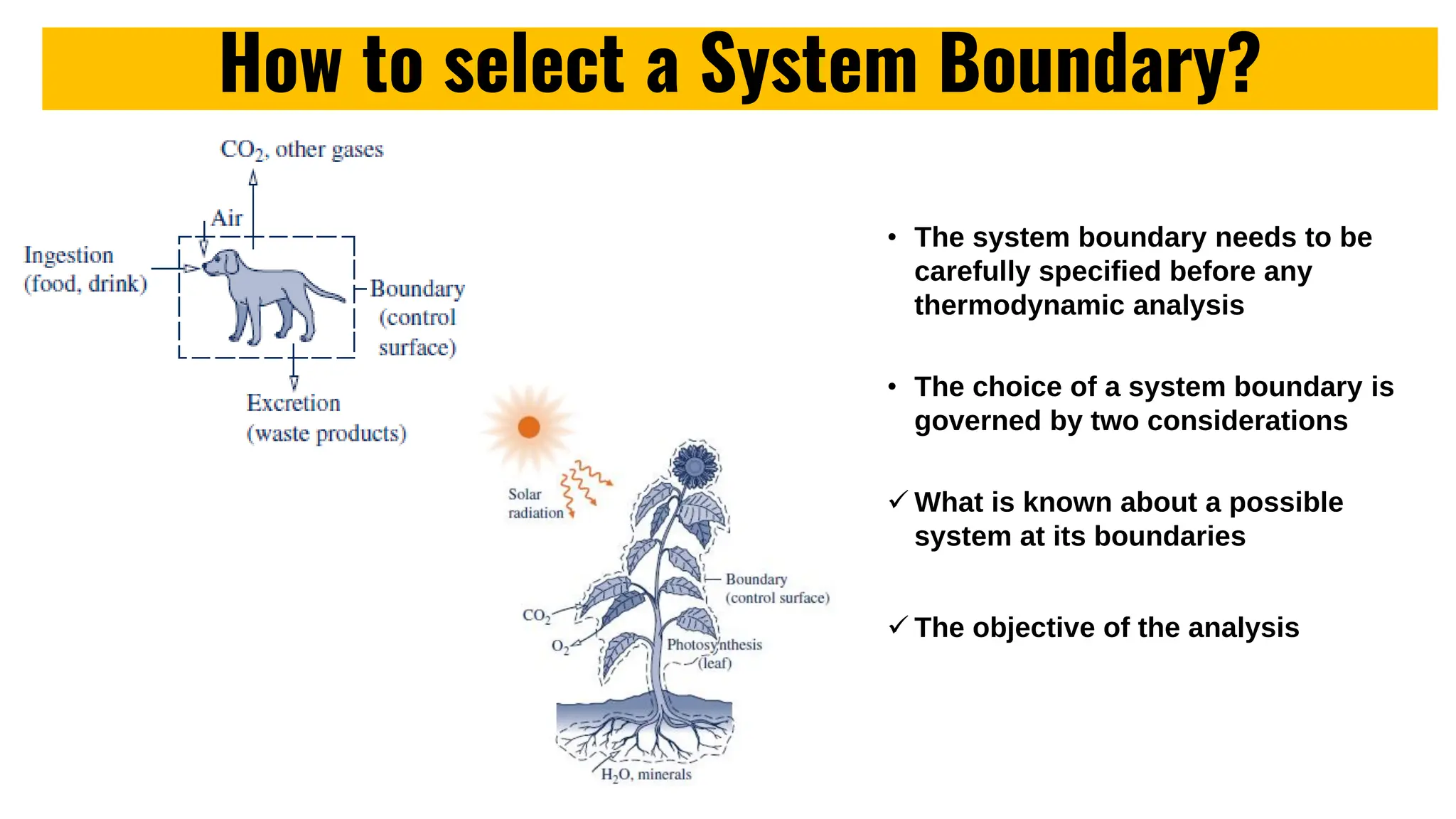
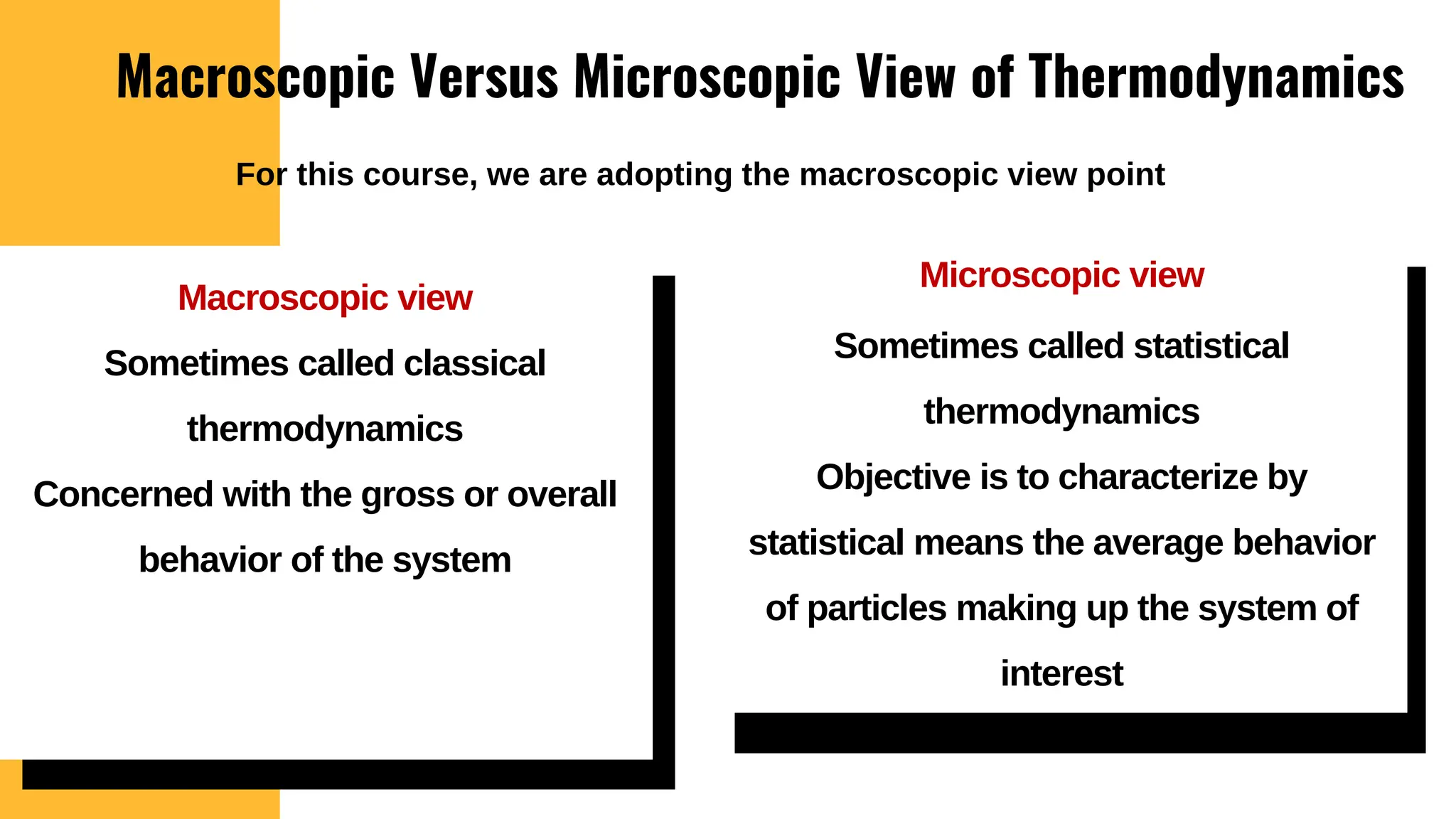
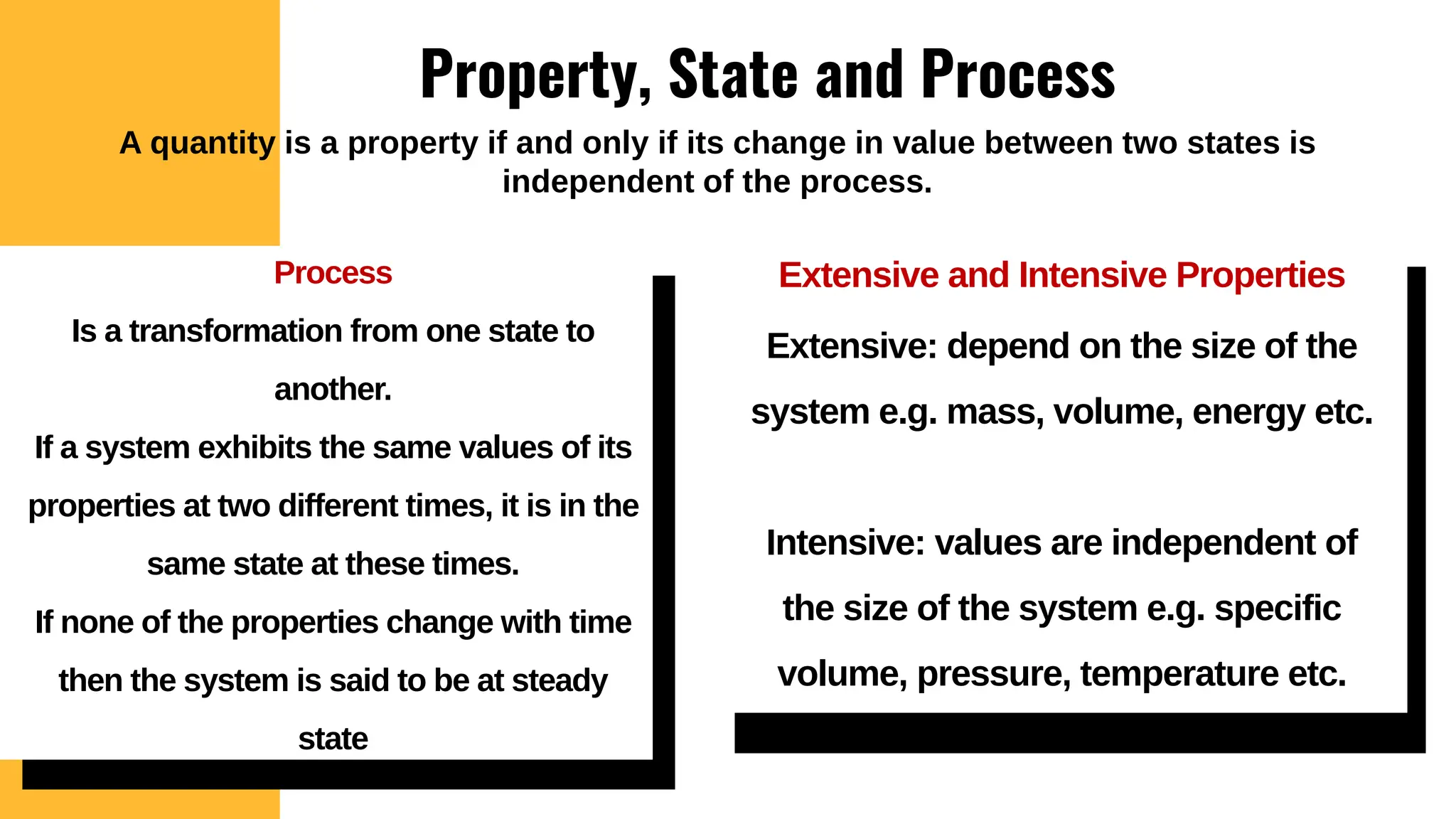
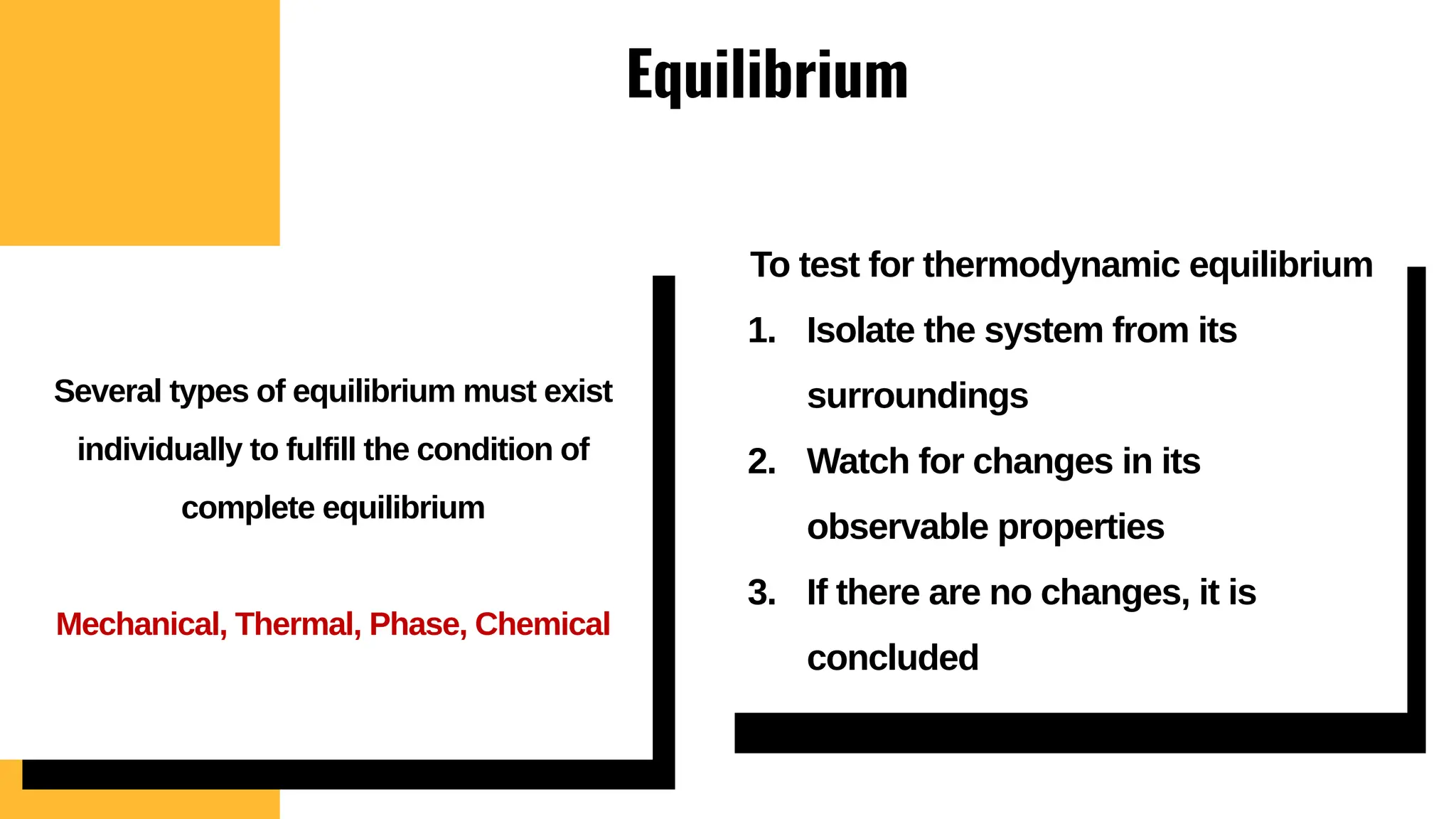
The document outlines the fundamentals of thermodynamics, emphasizing its importance in addressing 21st-century challenges like energy efficiency and climate change. It defines key concepts such as closed and open systems, properties, and processes while detailing various application areas in engineering. Additionally, it contrasts macroscopic and microscopic views of thermodynamics, focusing on the role of system boundaries and the concept of equilibrium.