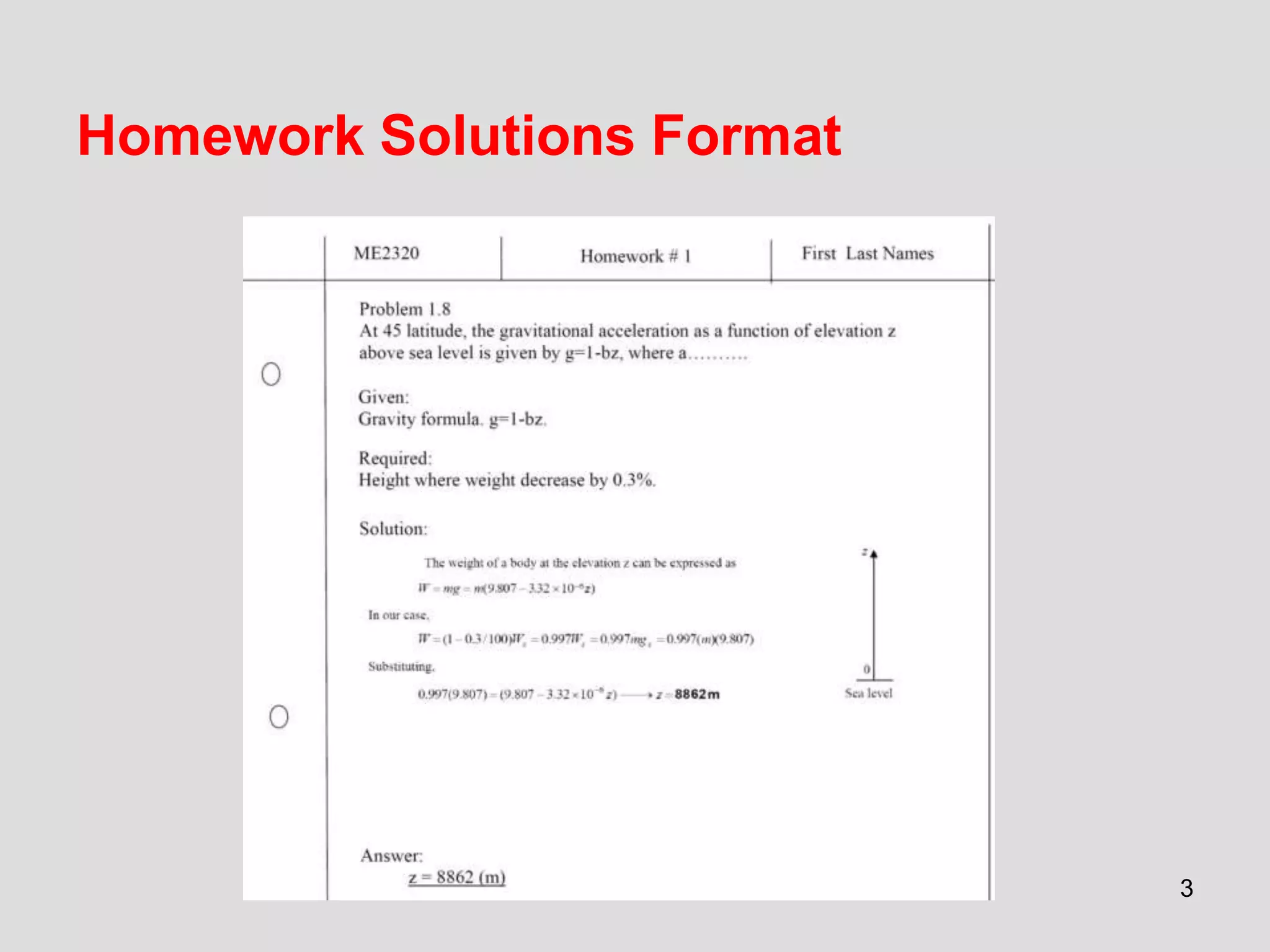
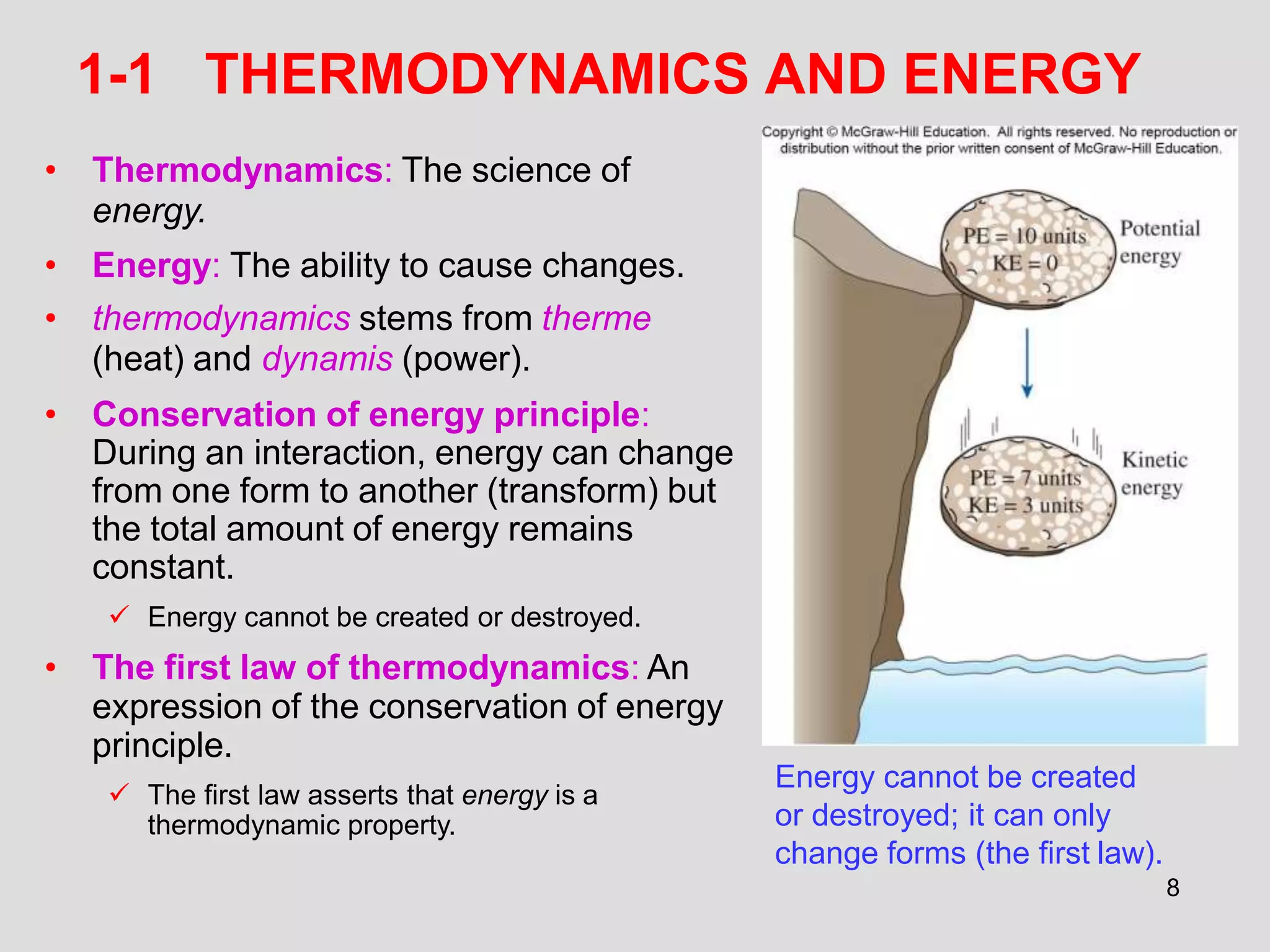
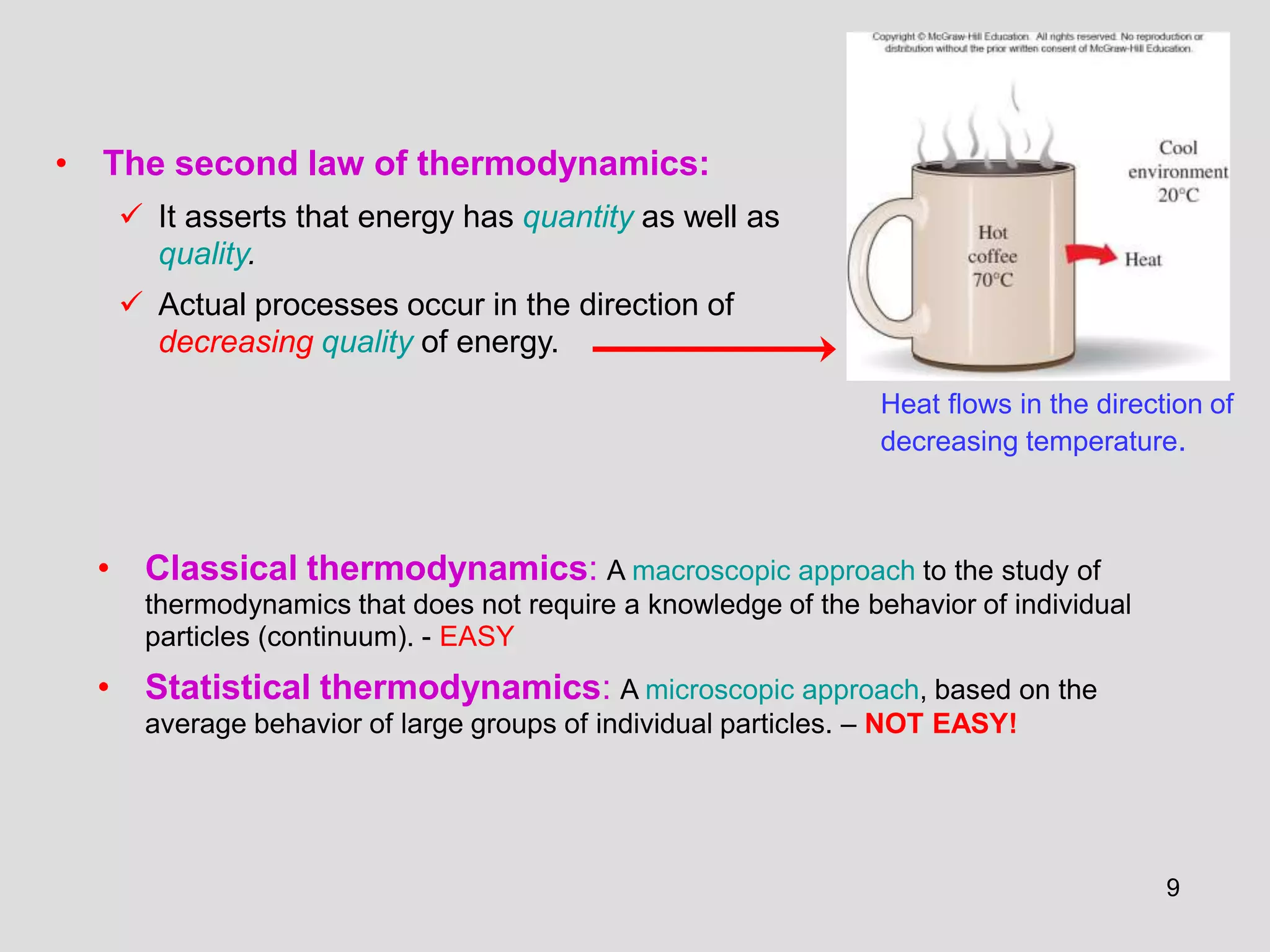
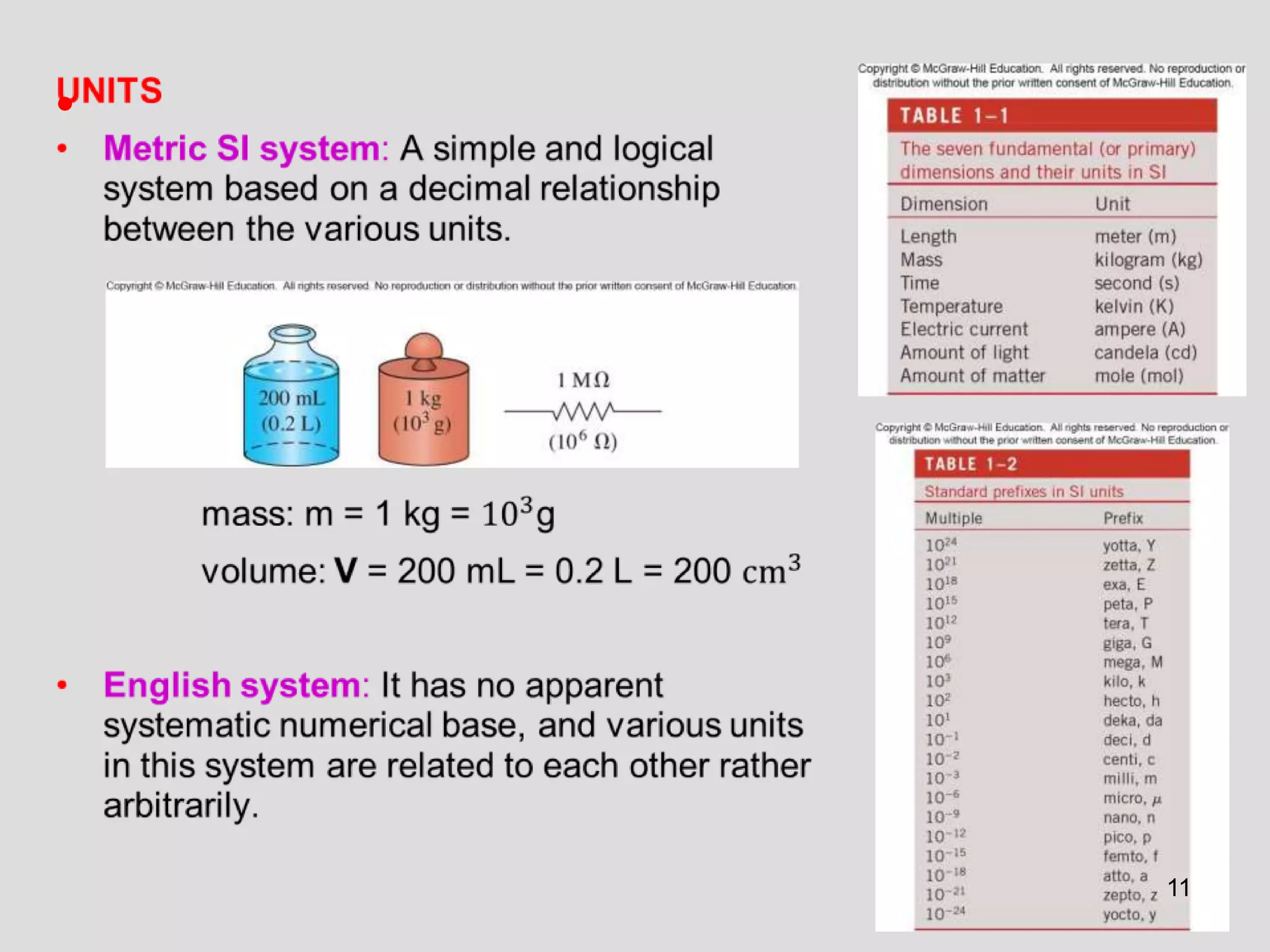
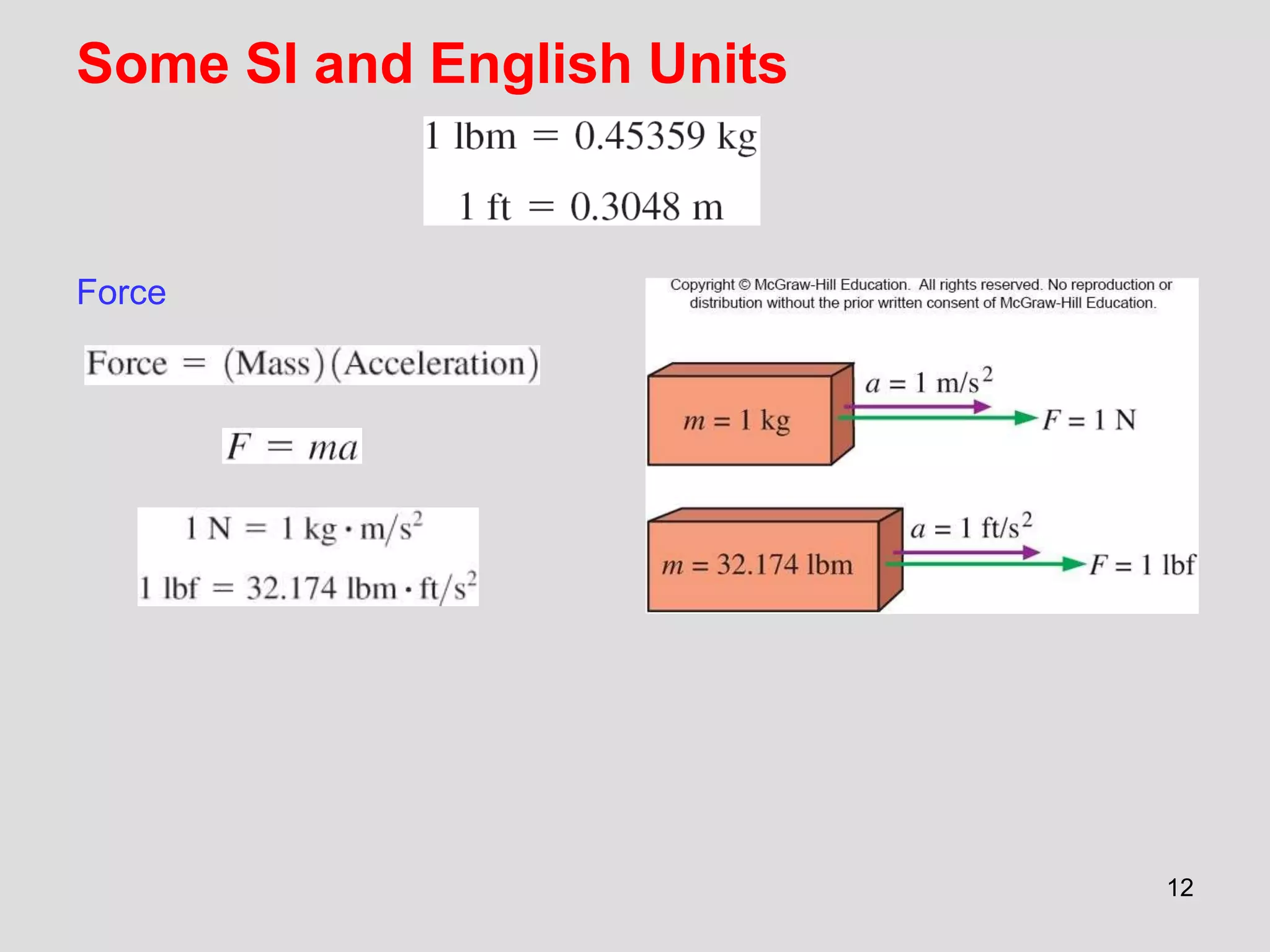
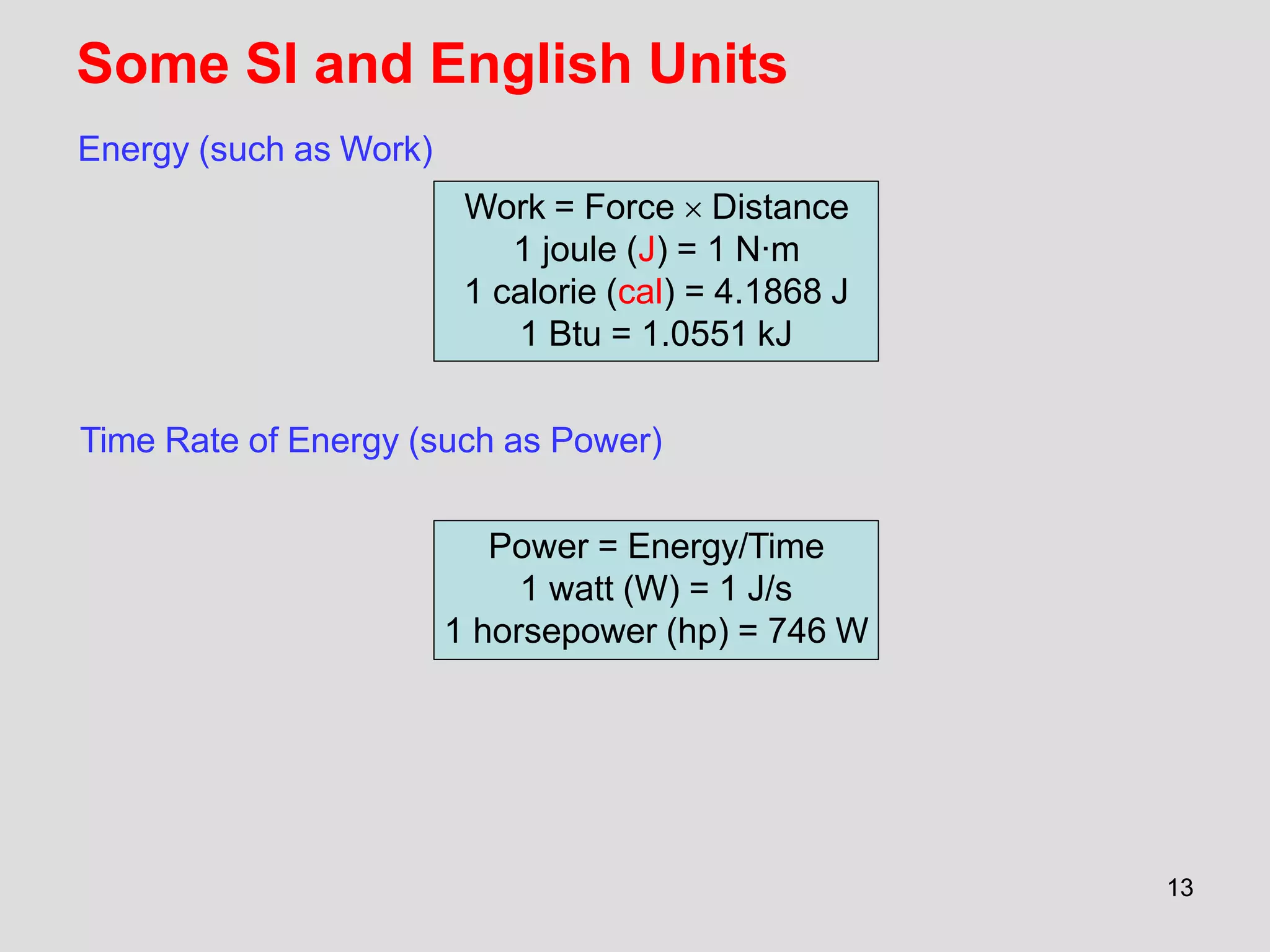
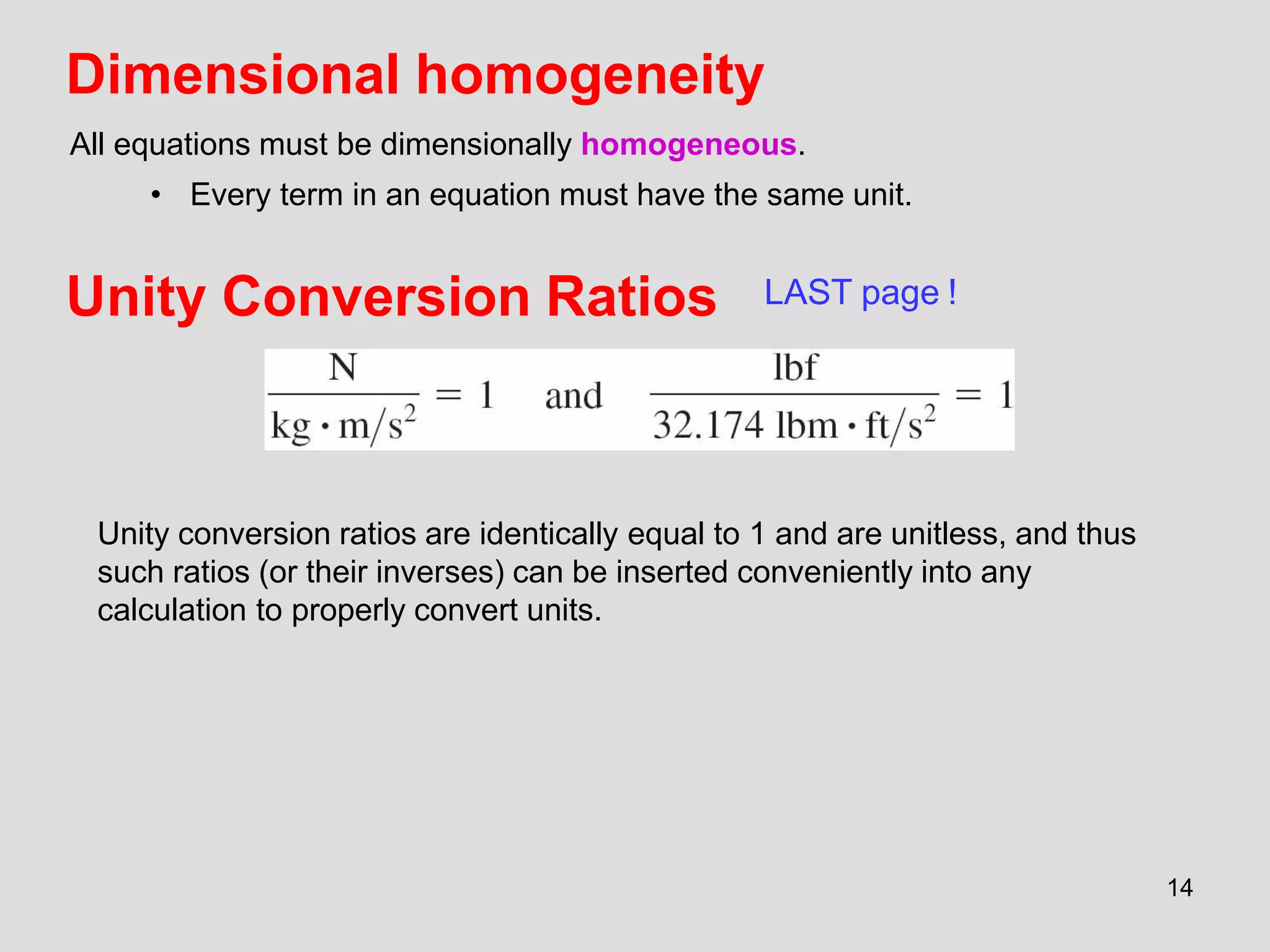
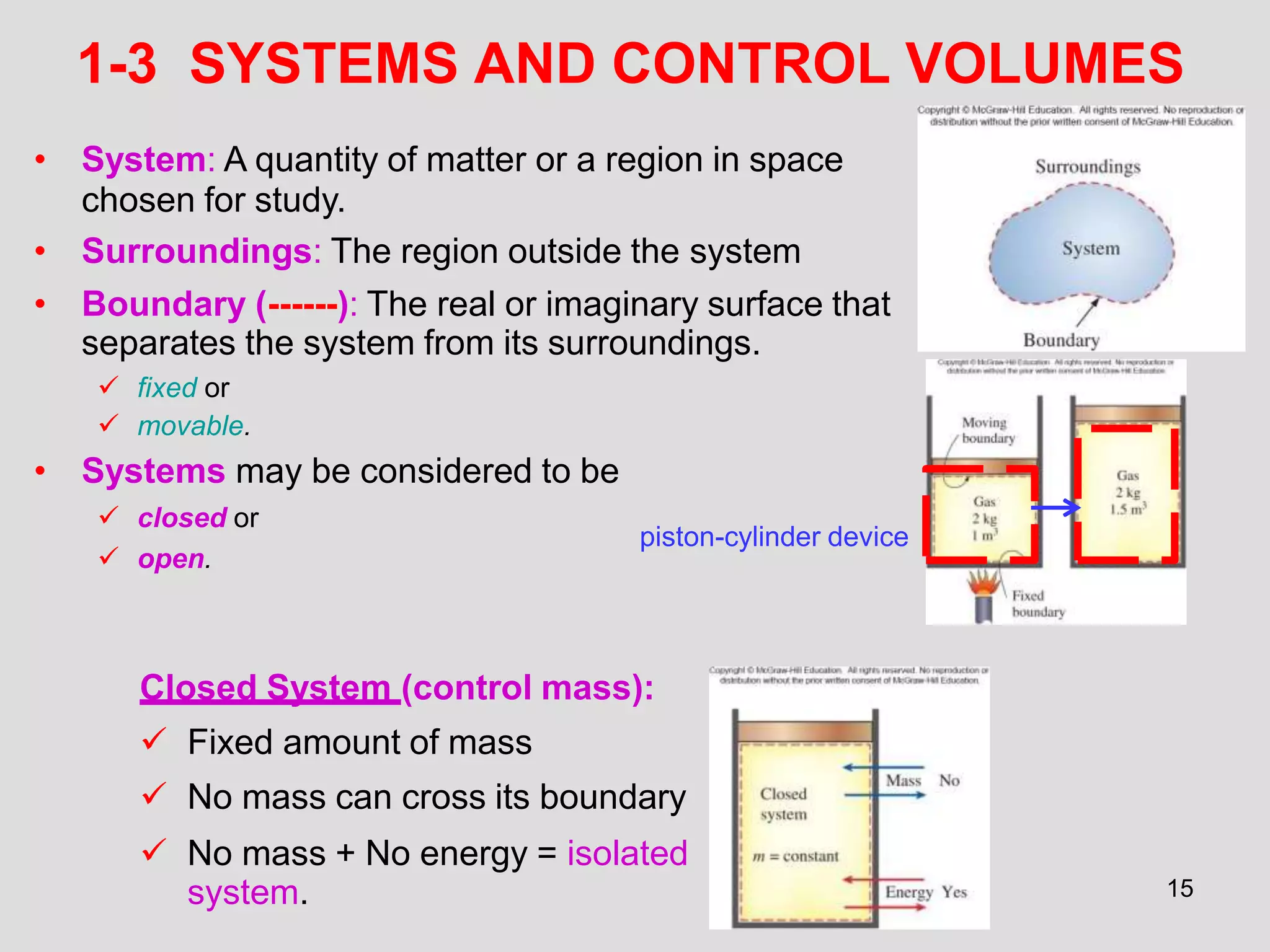
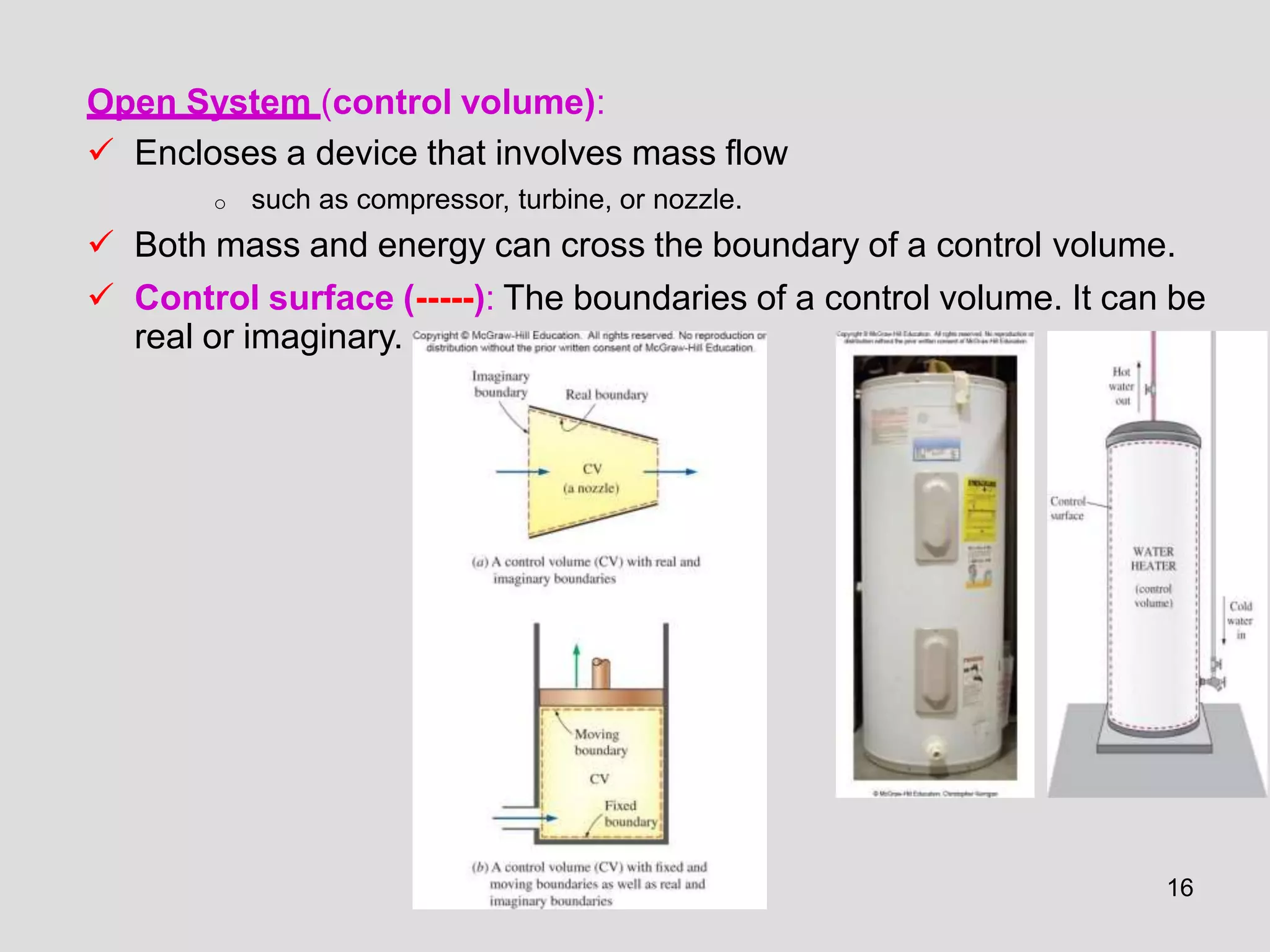
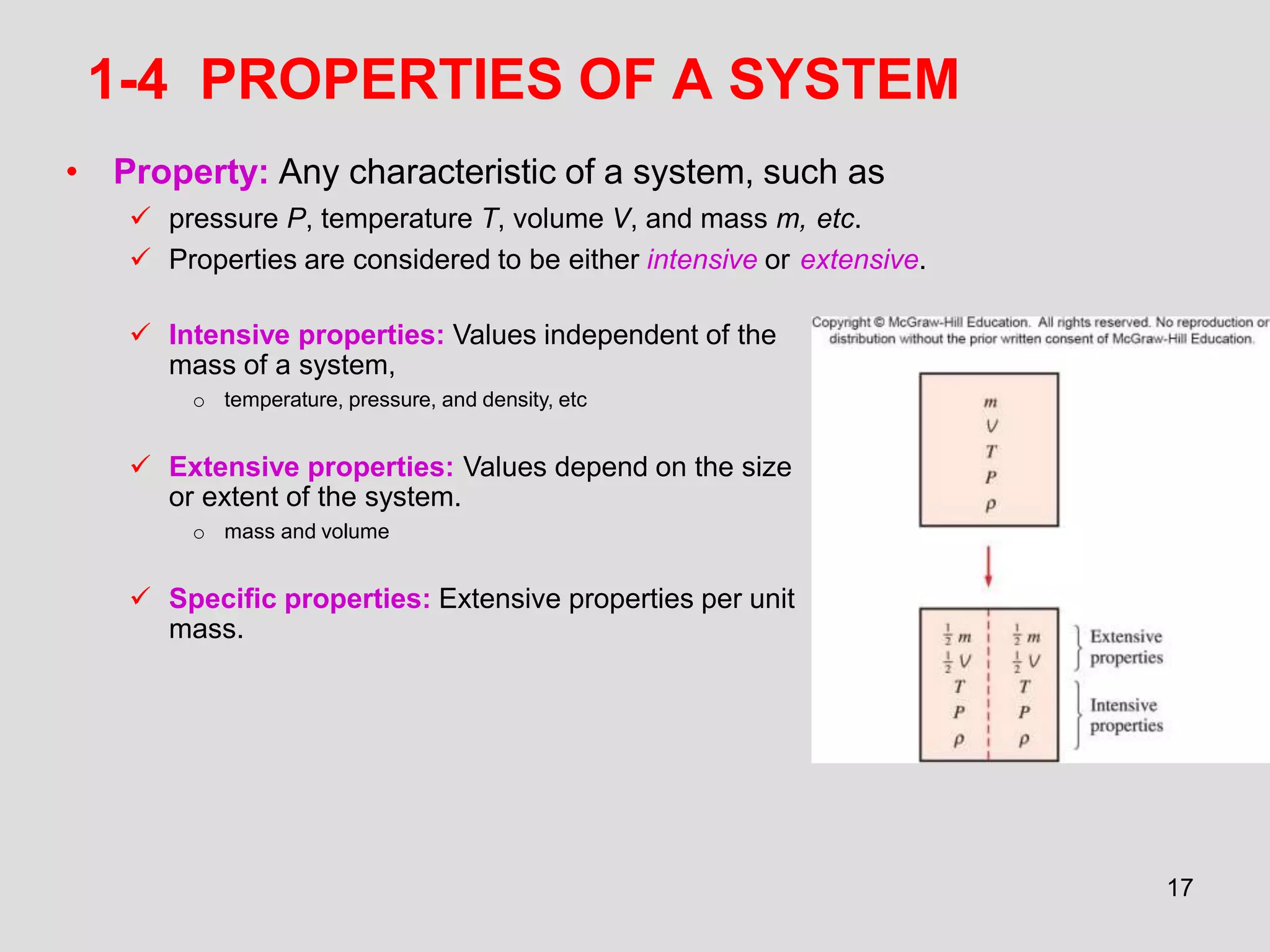
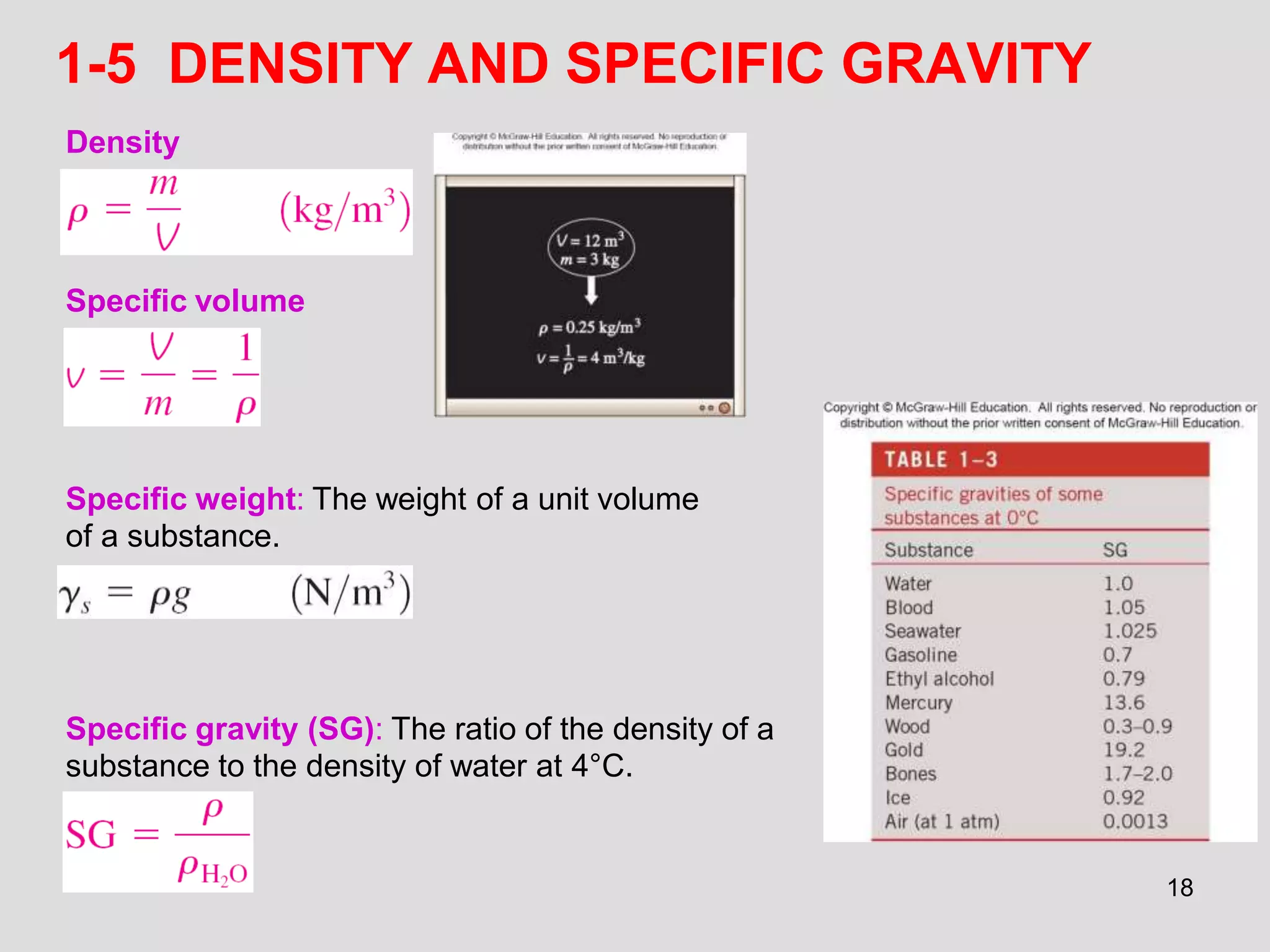
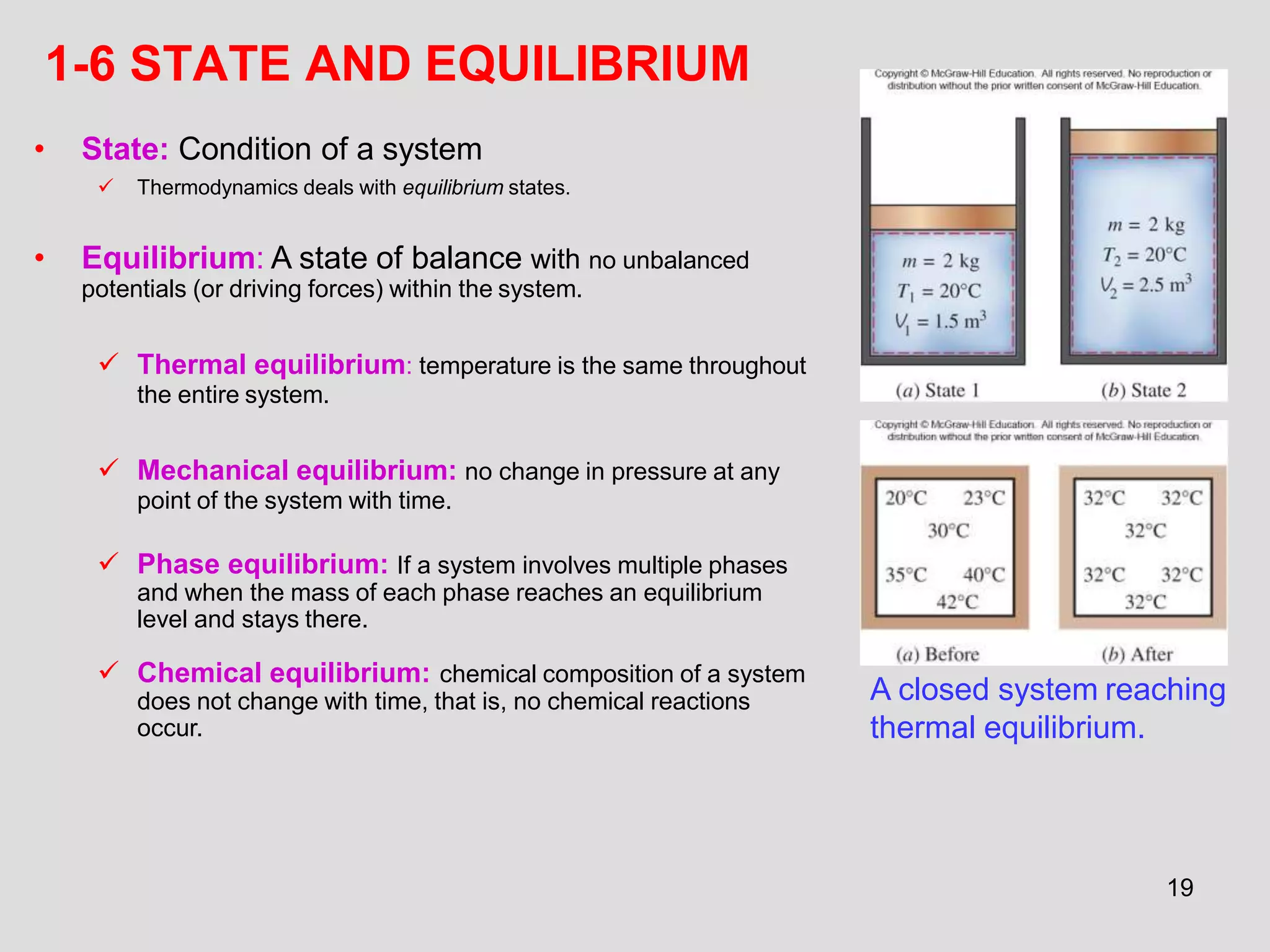
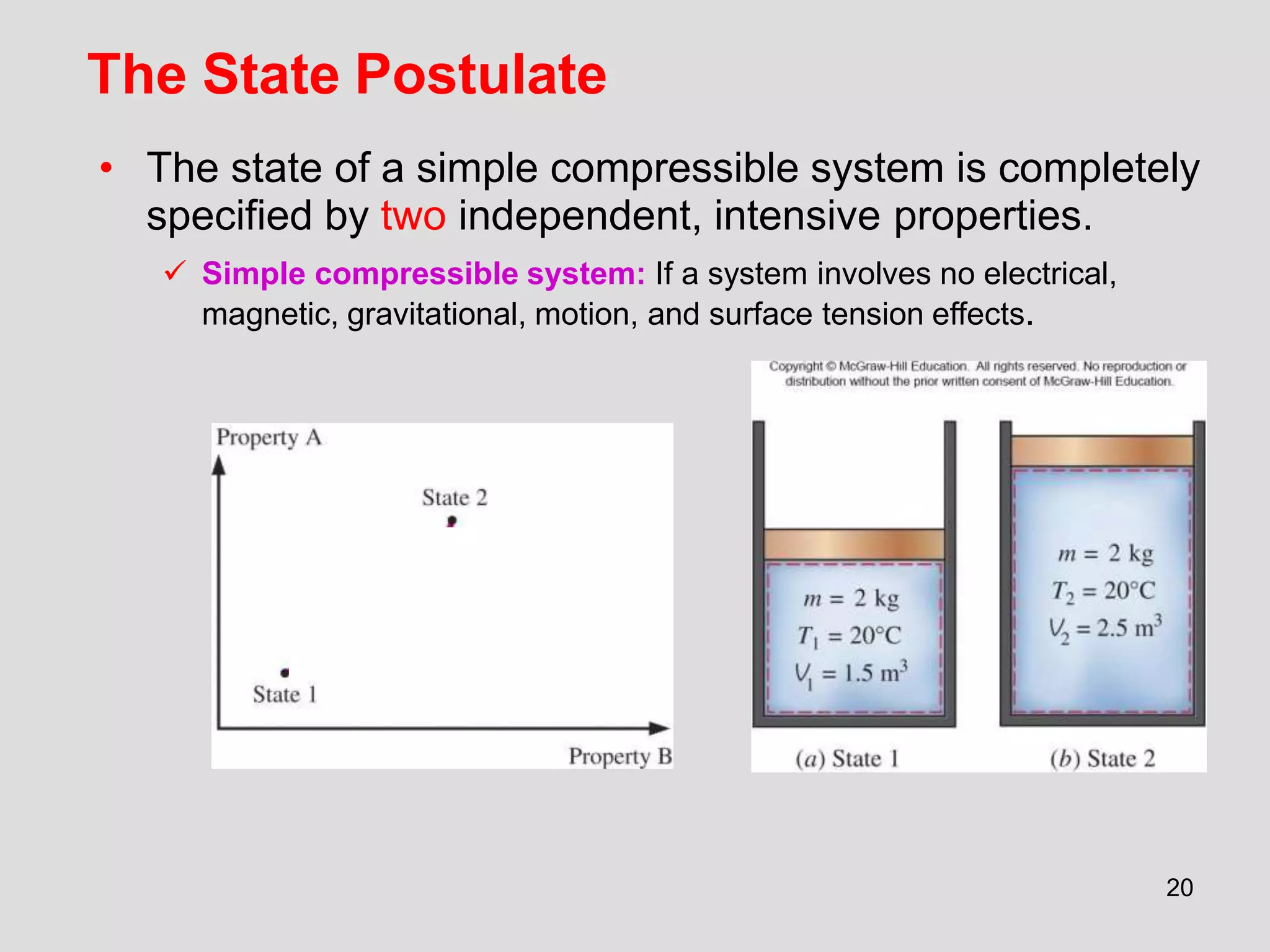
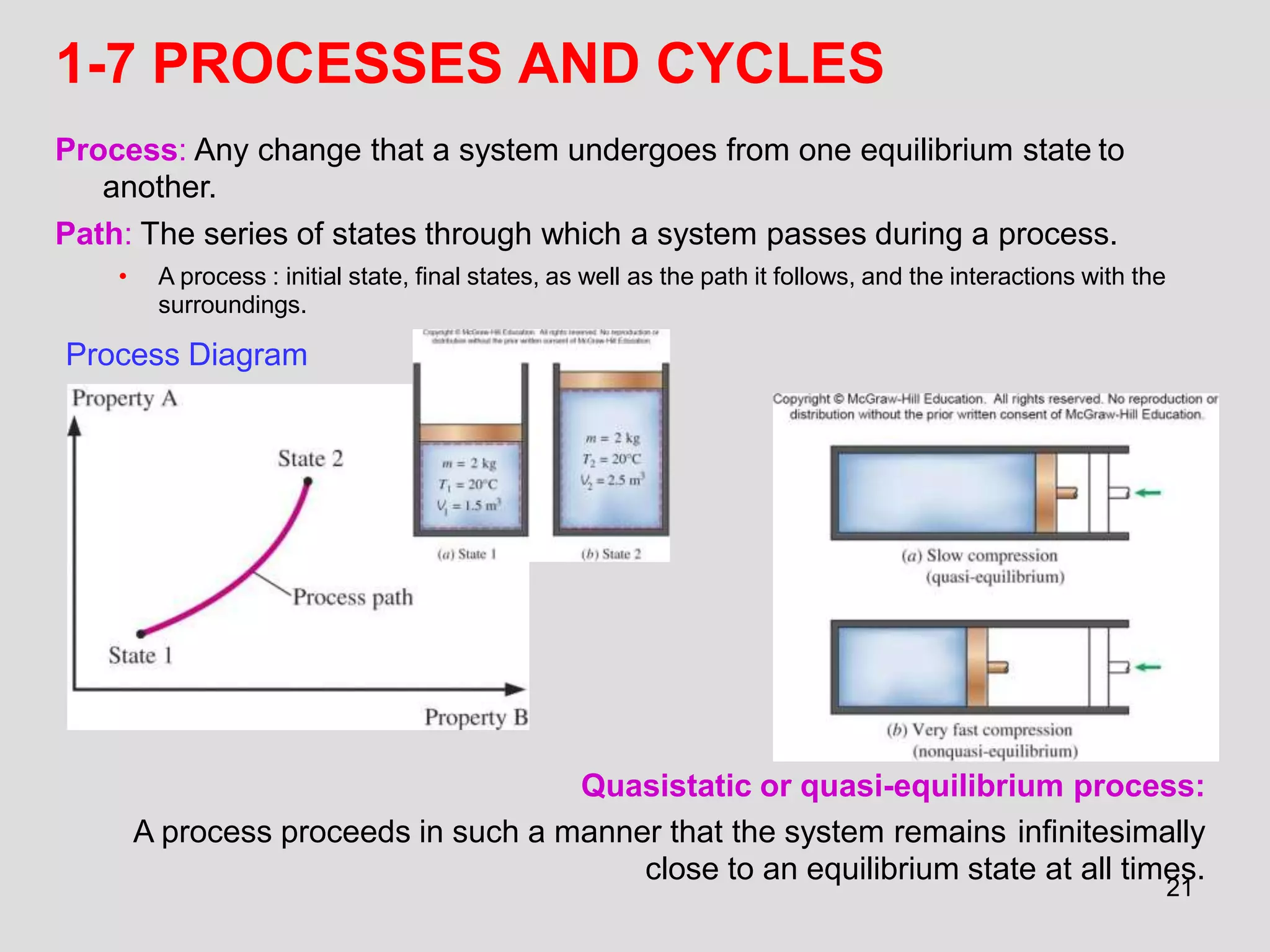
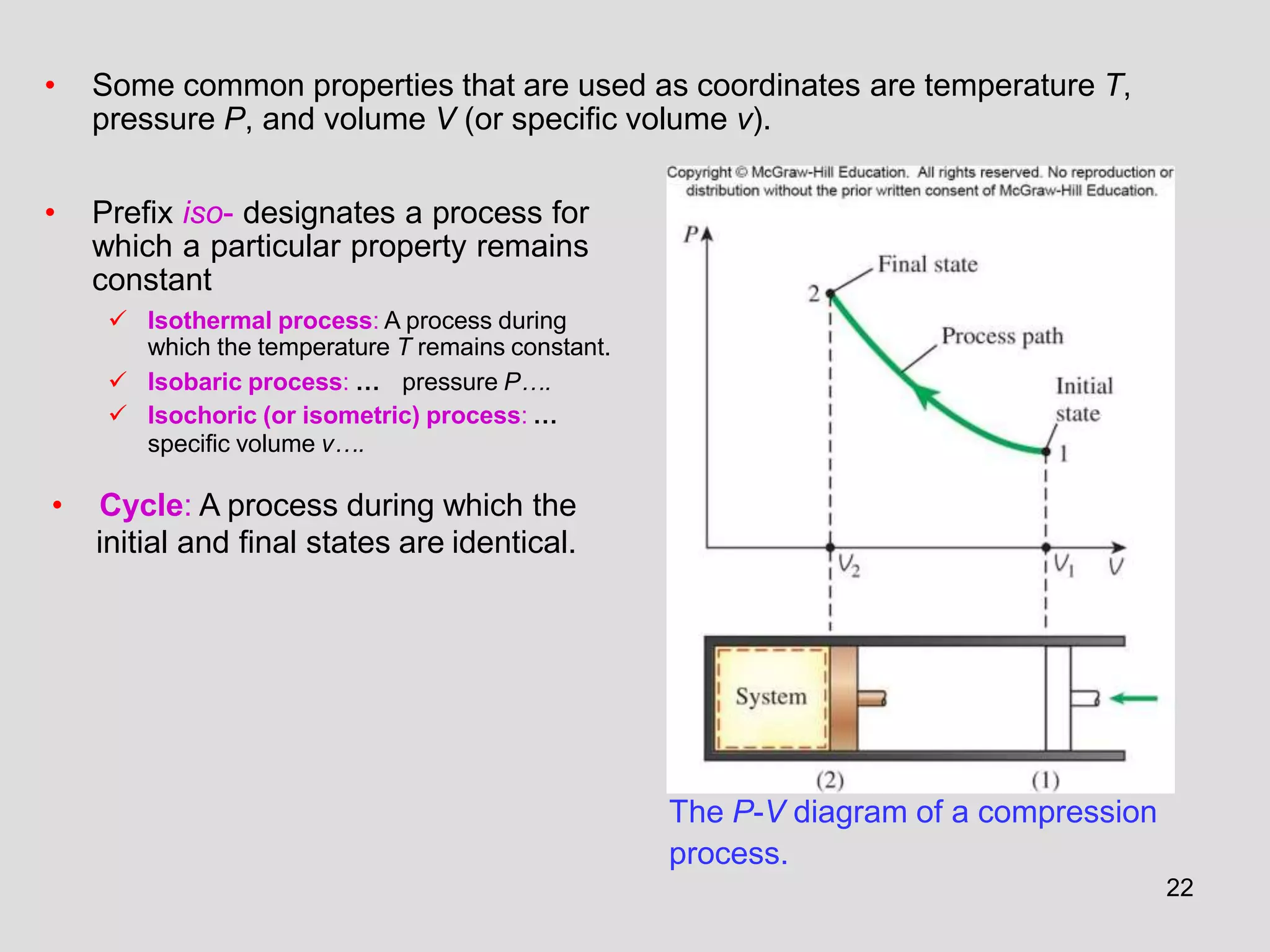
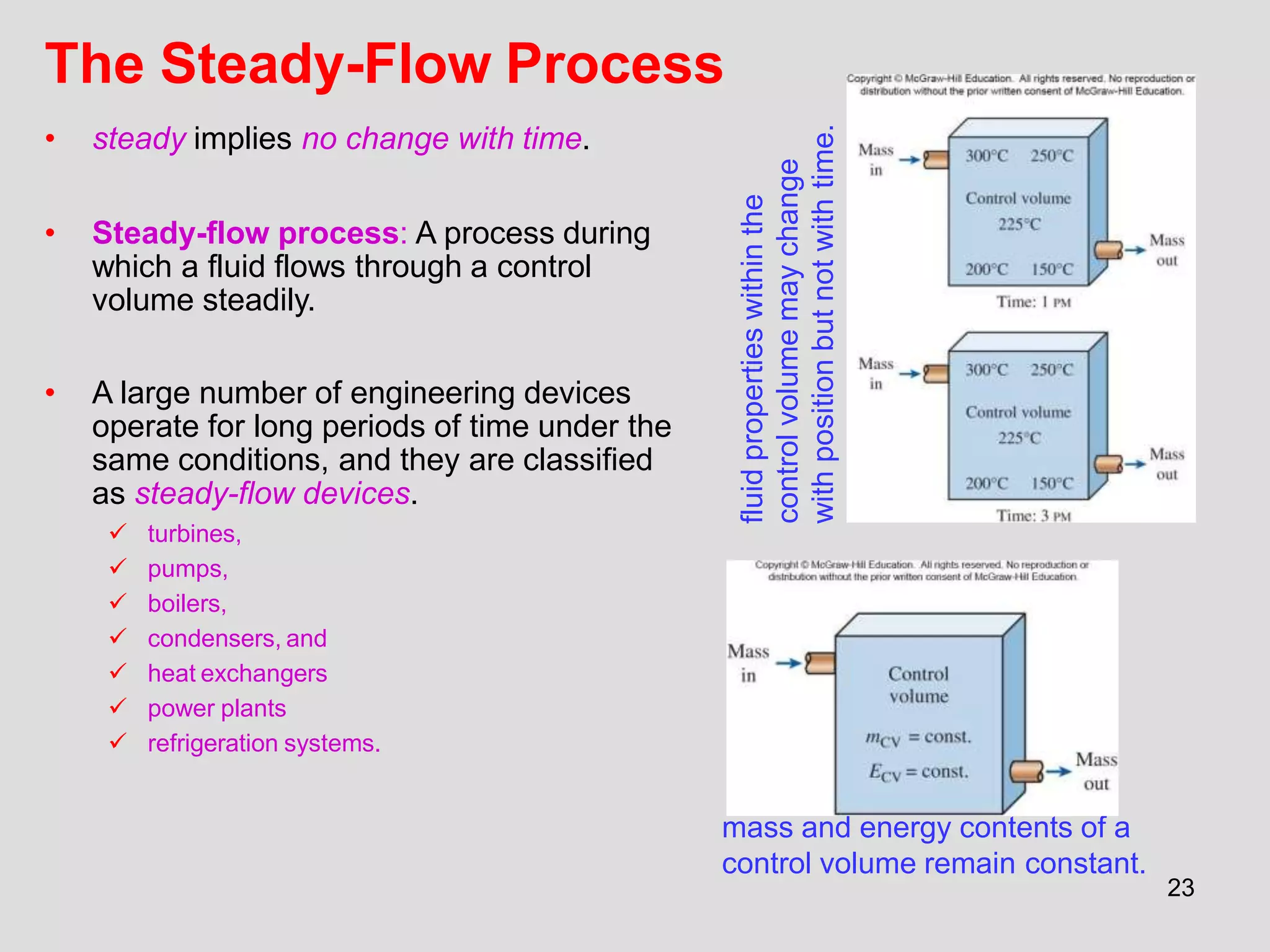
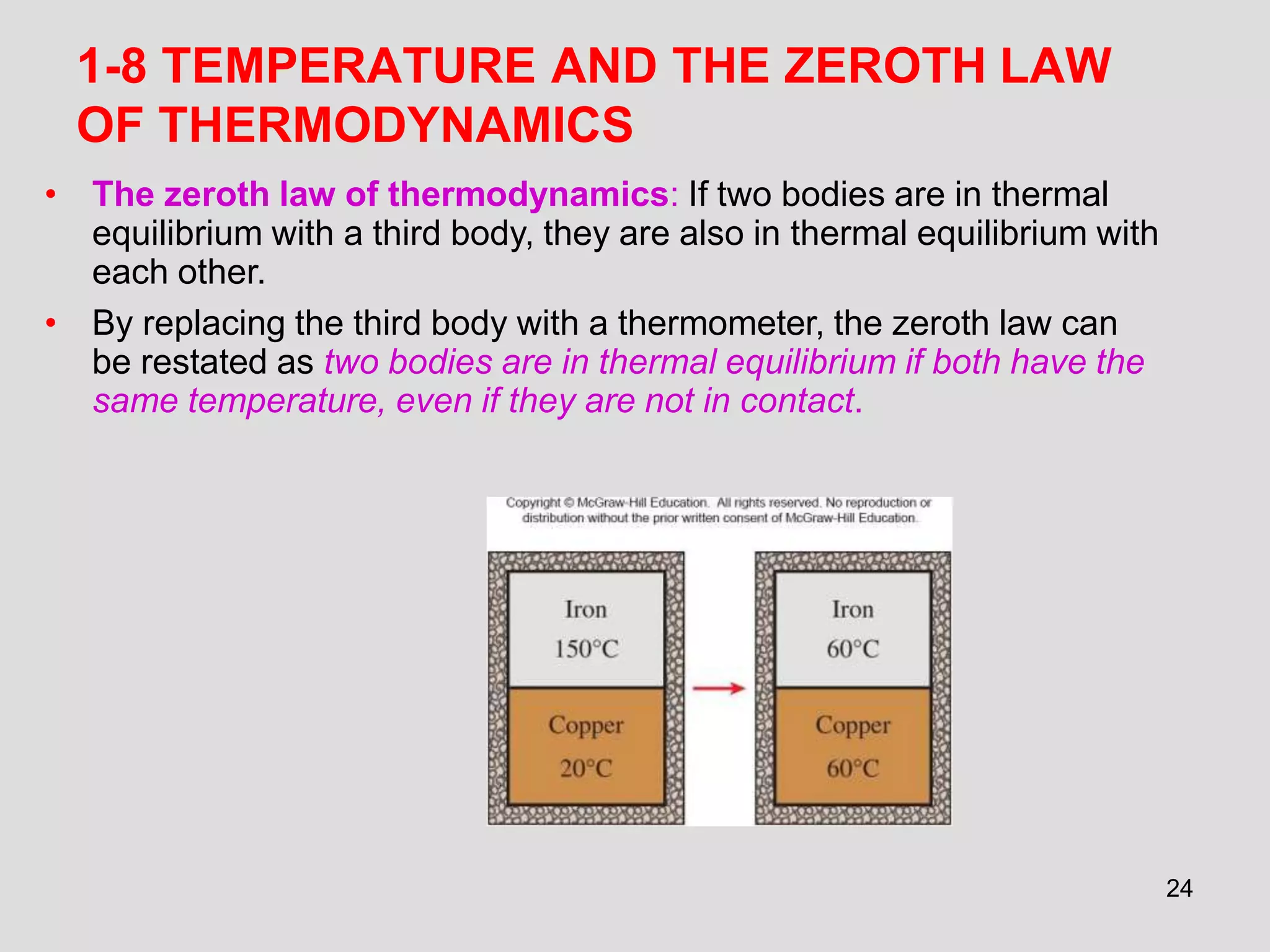
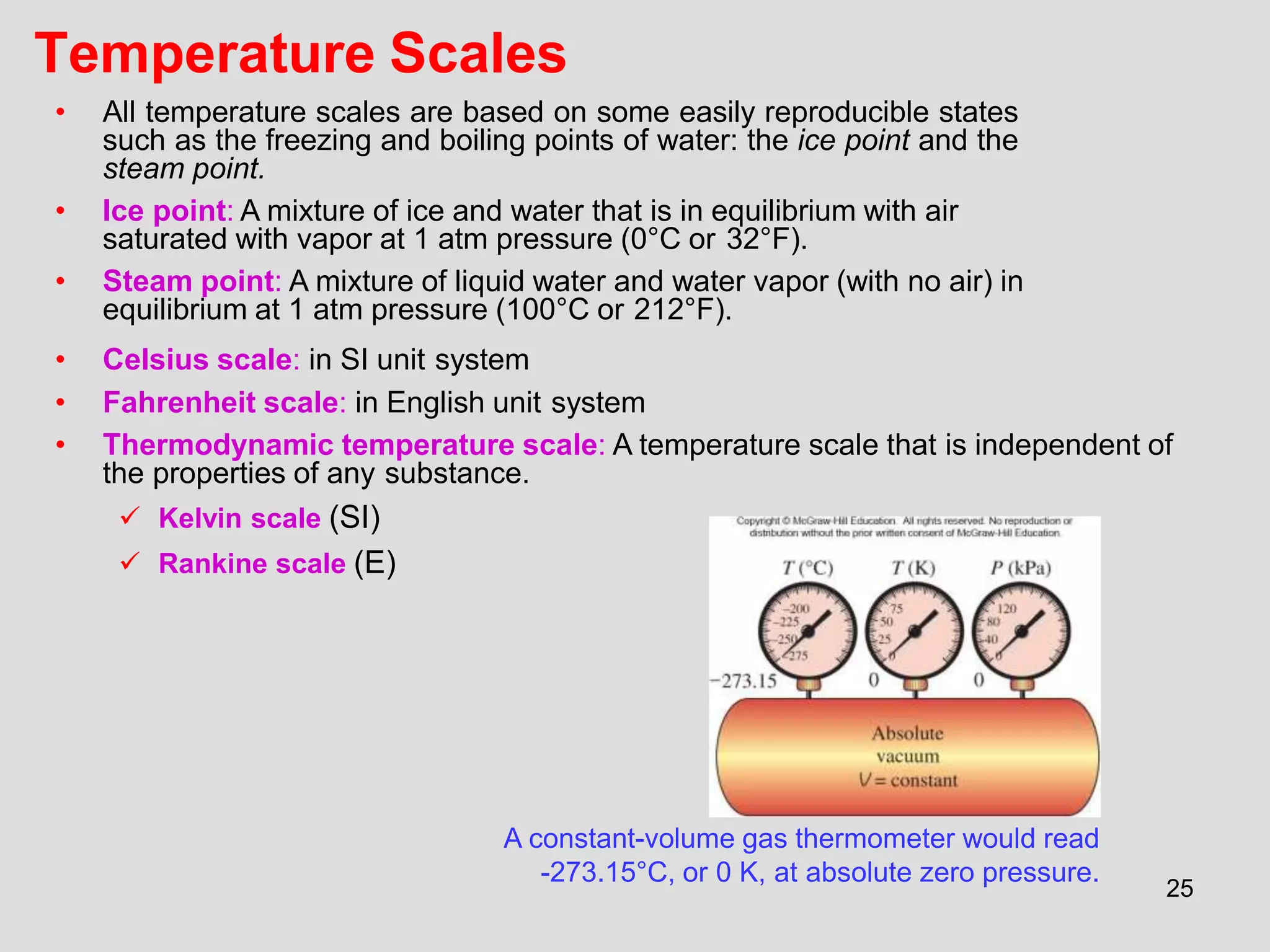
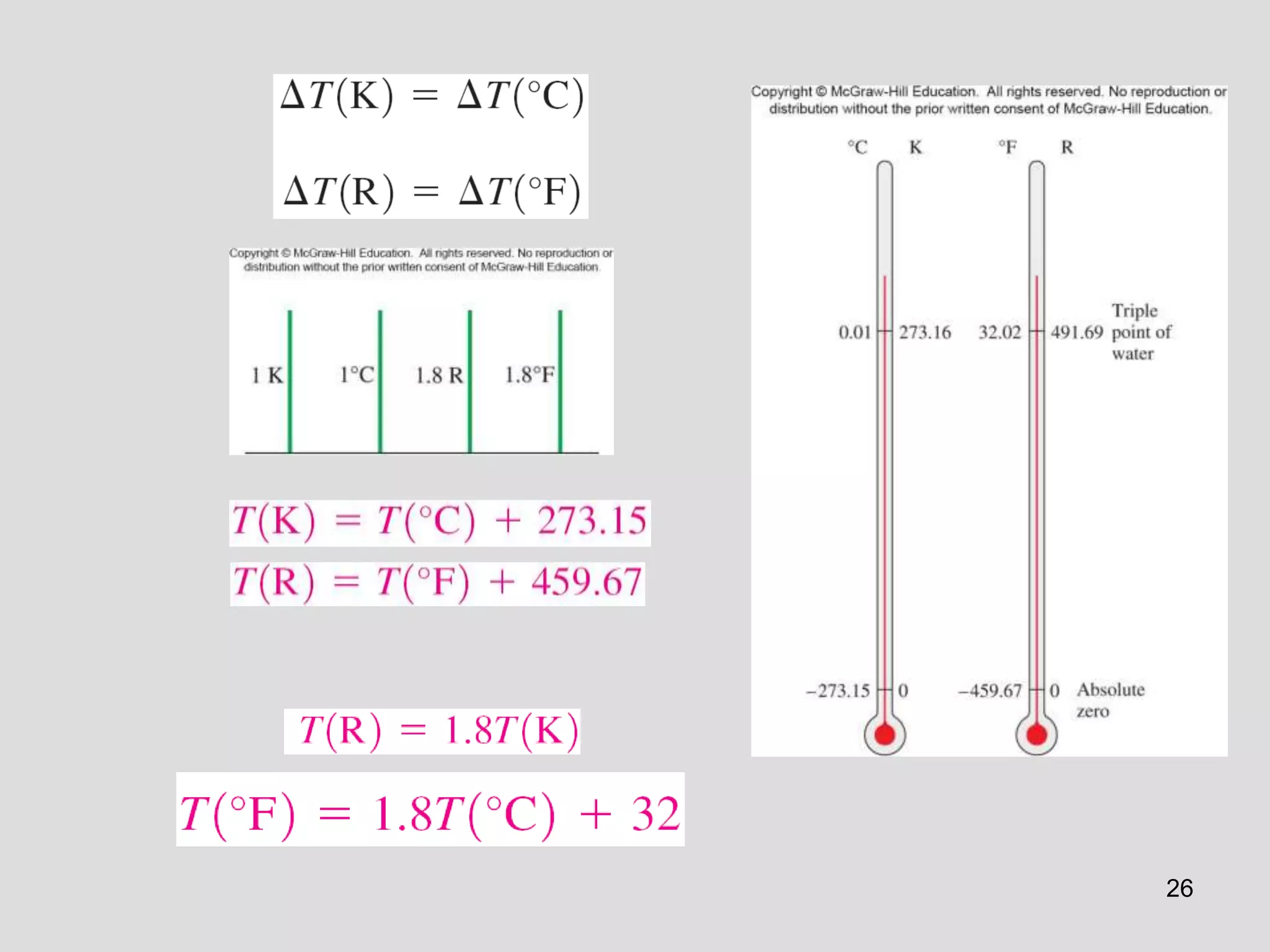
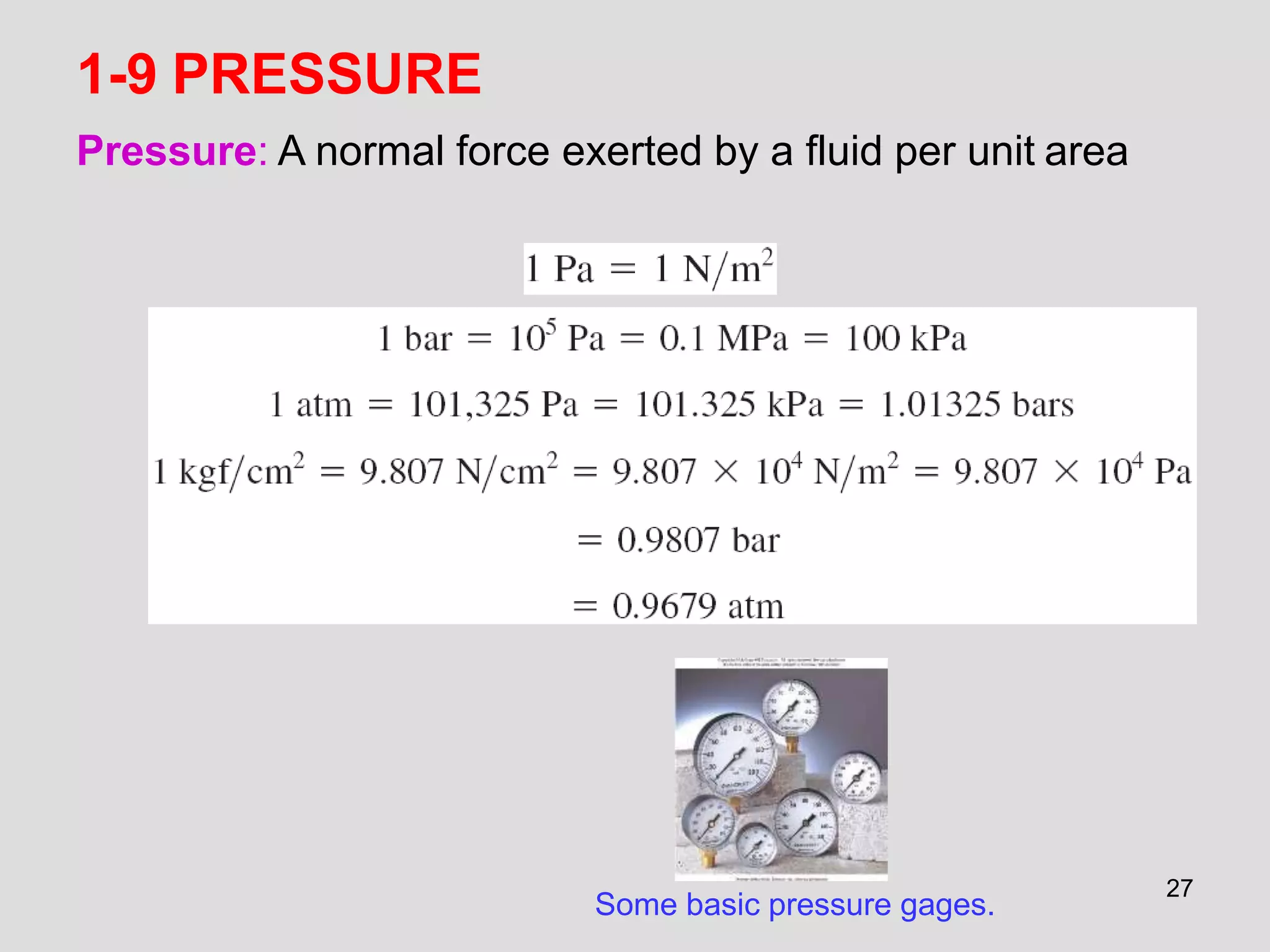
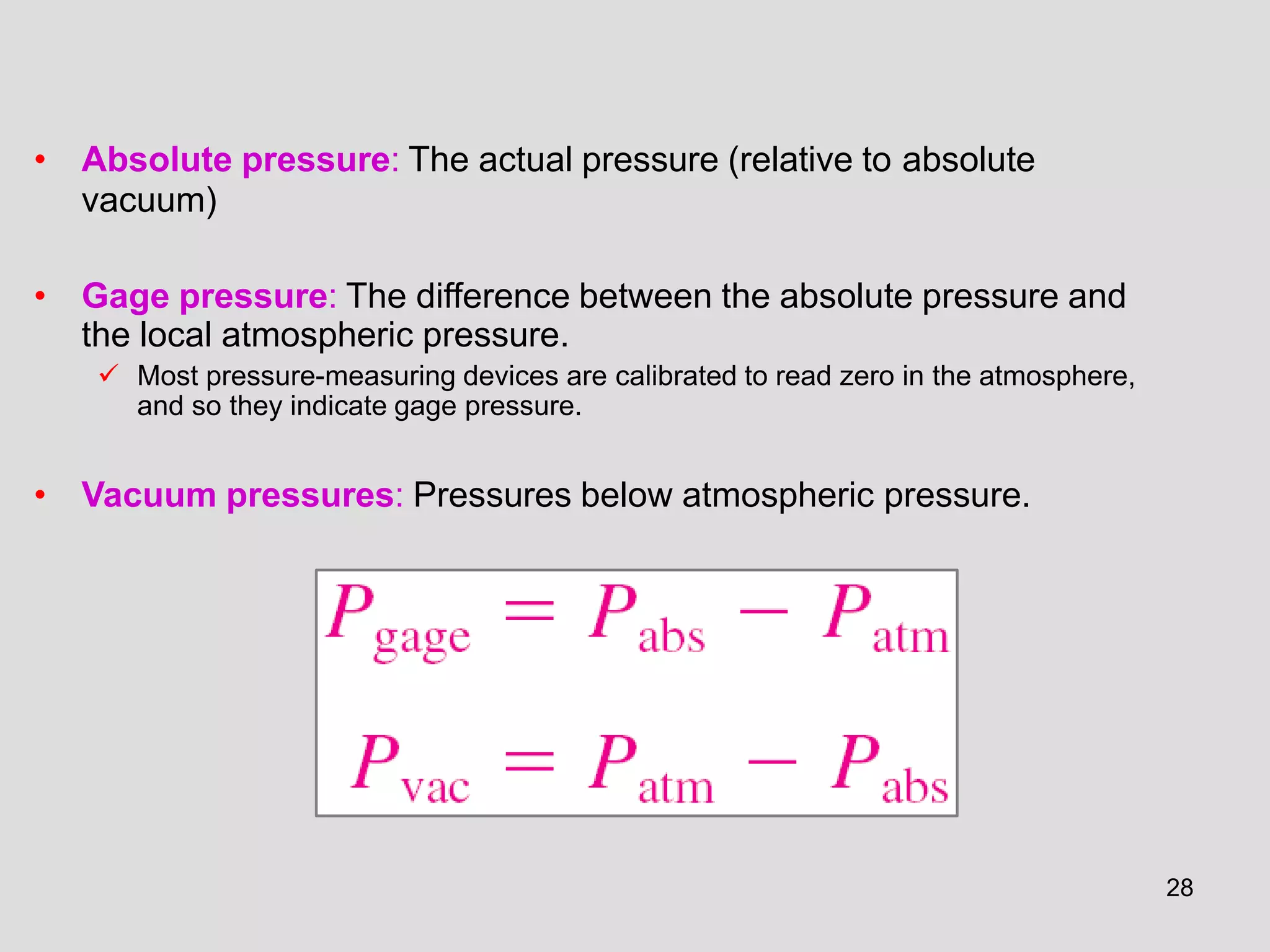
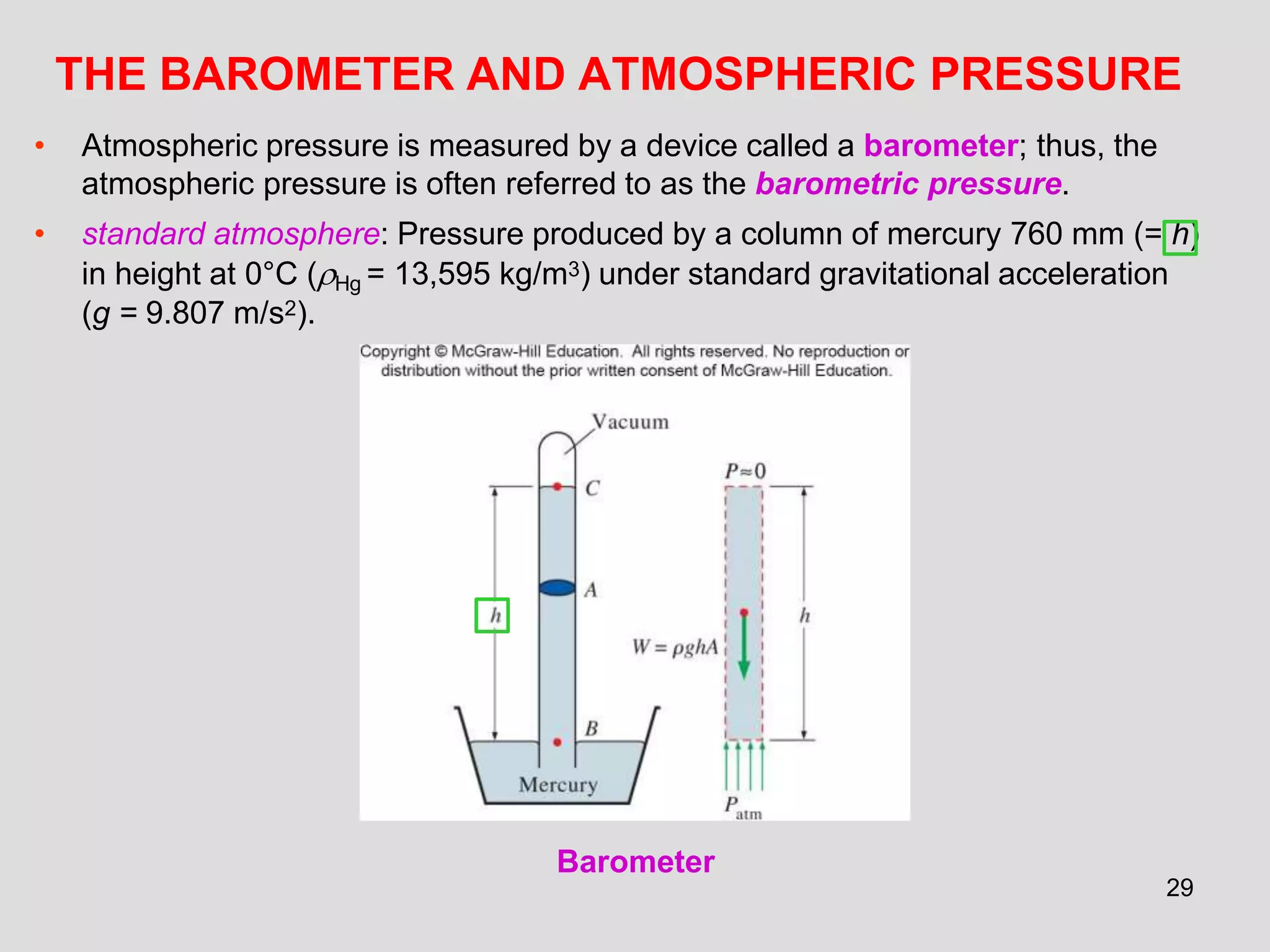
This document provides an overview of the ME2320 Thermodynamics I course for Summer I 2016, including the instructor details, syllabus, homework format, and chapter outlines. The first chapter introduces fundamental thermodynamics concepts like systems, properties, processes, the laws of thermodynamics, and temperature and pressure units and scales. It emphasizes developing problem-solving skills through a systematic 7-step technique.